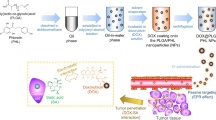
Abstract
In this study, we synthesized PLA-PEI micelles which was co-loaded with an anticancer drug, camptothecin (CPT), and survivin-shRNA (sur-shRNA). The hydrophobic CPT was encapsulated in the core of the polymeric micelles while sur-shRNA was adsorbed on the shell of the cationic micelles. Then, the positively-charged sur-shRNA-loaded micelles were coated with poly carboxylic acid dextran (PCAD) to form PLA/PEI-CPT-SUR-DEX. To selectively target the system to colon cancer cells, AS1411 aptamer was covalently attached to the surface of the PCAD-coated nanoparticles (PLA/PEI-CPT-SUR-DEX-APT). PLA/PEI-CPT-SUR-DEX-APT enhanced cellular uptake through receptor-mediated endocytosis followed by increased CPT accumulation, downregulation of survivin, and thereby 38% cell apoptosis. In C26 tumor-bearing mice models, after administered intravenously, PLA/PEI-CPT-SUR-DEX-APT and PLA/PEI-CPT-SUR-DEX formulations resulted in a significant inhibition of the tumor growth with tumor inhibition rate of 93% and 87%, respectively. Therefore, PLA/PEI-CPT-SUR-DEX-APT could be a versatile co-delivery vehicle for promising therapy of colorectal cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Xiao B, Si X, Han MK, Viennois E, Zhang M, Merlin D. Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J Mater Chem B. 2015;3:7724–33.
Teo PY, Cheng W, Hedrick JL, Yang YY. Co-delivery of drugs and plasmid DNA for cancer therapy. Adv Drug Deliv Rev. 2016;98:41–63.
Zhonghong L, Lianjie L, Changqing Z, Ying H, Yu J, Yan L. The influence of survivin shRNA on the cell cycle and the invasion of SW480 cells of colorectal carcinoma. J Exp Clin Cancer Res. 2008;27:20.
Wang K, Kievit FM, Zhang M. Nanoparticles for cancer gene therapy: Recent advances, challenges, and strategies. Pharmacol Res. 2016;114:56–66.
Bi Y, Lee RJ, Wang X, Sun Y, Wang M, Li L, et al. Liposomal codelivery of an SN38 prodrug and a survivin siRNA for tumor therapy. Int J Nanomedicine. 2018;13:5811–22.
Yan X, Yu Q, Guo L, Guo W, Guan S, Tang H, et al. Positively charged combinatory drug delivery systems against multidrug resistant breast cancer: beyond the drug combination. ACS Appl Mater Interfaces. 2017;9:6804–15.
Shim G, Kim M-G, Park JY, Oh Y-K. Application of cationic liposomes for delivery of nucleic acids. Asian J Pharm Sci. 2013;8:72–80.
Son S, Kim WJ. Biodegradable nanoparticles modified by branched polyethylenimine for plasmid DNA delivery. Biomater. 2010;31:133–43.
Zhu J, Tang A, Law LP, Feng M, Ho KM, Lee DK, et al. Amphiphilic core–shell nanoparticles with poly (ethylenimine) shells as potential gene delivery carriers. Bioconjug Chem. 2005;16:139–46.
Park YM, Shin BA, Oh IJ. Poly(L-lactic acid)/polyethylenimine nanoparticles as plasmid DNA carriers. Arch Pharm Res. 2008;31:96–102.
Mandal H, Katiyar SS, Swami R, Kushwah V, Katare PB, Meka AK, et al. ε-Poly-L-Lysine/plasmid DNA nanoplexes for efficient gene delivery in vivo. Int J Pharm. 2018;542:142–52.
Shen W, Wang R, Fan Q, Gao X, Wang H, Shen Y, et al. Natural polyphenol inspired polycatechols for efficient siRNA delivery. CCS Chem. 2020;2:146–57.
Nam JP, Kim S, Kim SW. Design of PEI-conjugated bio-reducible polymer for efficient gene delivery. Int J Pharm. 2018;545:295–305.
Chang Y-C, Chu IM. Methoxy poly(ethylene glycol)-b-poly(valerolactone) diblock polymeric micelles for enhanced encapsulation and protection of camptothecin. Eur Polym J. 2008;44:3922–30.
Torchilin VP. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell Mol Life Sci. 2004;61:2549–59.
Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine. 2010;6:714–29.
Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Delivery Rev. 2012;64:37–48.
Date T, Nimbalkar V, Kamat J, Mittal A, Mahato RI, Chitkara D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J Control Release. 2018;271:60–73.
Praetorius NP, Mandal TK. Engineered nanoparticles in cancer therapy. Recent Pat Drug Deliv Formul. 2007;1:37–51.
Kunii R, Onishi H, Ueki K-i, Koyama K-i, Machida Y. Particle characteristics and biodistribution of camptothecin-loaded PLA/(PEG-PPG-PEG) nanoparticles. Drug Deliv. 2008;15:3–10.
Danafar H. MPEG–PCL copolymeric nanoparticles in drug delivery systems. Cogent Med. 2016;3:1142411.
Yu M, Huang S, Yu KJ, Clyne AM. Dextran and polymer polyethylene glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture. Int J Mol Sci. 2012;13:5554–70.
Bilensoy E. Cationic nanoparticles for cancer therapy. Expert Opin Drug Deliv. 2010;7:795–809.
Gu Q, Xing JZ, Huang M, He C, Chen J. SN-38 loaded polymeric micelles to enhance cancer therapy. Nanotechnology. 2012;23:205101.
Xu W, Siddiqui IA, Nihal M, Pilla S, Rosenthal K, Mukhtar H, et al. Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer. Biomaterials. 2013;34:5244–53.
Aravind A, Jeyamohan P, Nair R, Veeranarayanan S, Nagaoka Y, Yoshida Y, et al. AS1411 aptamer tagged PLGA-lecithin-PEG nanoparticles for tumor cell targeting and drug delivery. Biotechnol Bioeng. 2012;109:2920–31.
Taghavi S, Ramezani M, Alibolandi M, Abnous K, Taghdisi SM. Chitosan-modified PLGA nanoparticles tagged with 5TR1 aptamer for in vivo tumor-targeted drug delivery. Cancer Lett. 2017;400:1–8.
Alibolandi M, Abnous K, Hadizadeh F, Taghdisi SM, Alabdollah F, Mohammadi M, et al. Dextran-poly lactide-co-glycolide polymersomes decorated with folate-antennae for targeted delivery of docetaxel to breast adenocarcinima in vitro and in vivo. J Control Release. 2016;241:45–56.
Khalkhali M, Sadighian S, Rostamizadeh K, Khoeini F, Naghibi M, Bayat N, et al. Synthesis and characterization of dextran coated magnetite nanoparticles for diagnostics and therapy. BioImpacts. 2015;5:141.
Yu C, Hu Y, Duan J, Yuan W, Wang C, Xu H, et al. Novel aptamer-nanoparticle bioconjugates enhances delivery of anticancer drug to MUC1-positive cancer cells in vitro. PloS ONE. 2011;6:e24077.
Alibolandi M, Taghdisi SM, Ramezani P, Hosseini Shamili F, Farzad SA, Abnous K, et al. Smart AS1411-aptamer conjugated pegylated PAMAM dendrimer for the superior delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. Int J Pharm. 2017;519:352–64.
Alibolandi M, Hoseini F, Mohammadi M, Ramezani P, Einafshar E, Taghdisi SM, et al. Curcumin-entrapped MUC-1 aptamer targeted dendrimer-gold hybrid nanostructure as a theranostic system for colon adenocarcinoma. Int J Pharm. 2018;549:67–75.
Shen S, Wu Y, Liu Y, Wu D. High drug-loading nanomedicines: progress, current status, and prospects. Int J Nanomedicine. 2017;12:4085.
Zhang Z, Wang X, Li B, Hou Y, Cai Z, Yang J, et al. Paclitaxel-loaded PLGA microspheres with a novel morphology to facilitate drug delivery and antitumor efficiency. RSC Adv. 2018;8:3274–85.
Tang S, Yin Q, Zhang Z, Gu W, Chen L, Yu H, et al. Co-delivery of doxorubicin and RNA using pH-sensitive poly (β-amino ester) nanoparticles for reversal of multidrug resistance of breast cancer. Biomaterials. 2014;35:6047–59.
Alibolandi M, Mohammadi M, Taghdisi SM, Ramezani M, Abnous K. Fabrication of aptamer decorated dextran coated nano-graphene oxide for targeted drug delivery. Carbohydr Polym. 2017;155:218–29.
Alibolandi M, Ramezani M, Abnous K, Hadizadeh F. AS1411 aptamer-decorated biodegradable polyethylene glycol–poly (lactic-co-glycolic acid) nanopolymersomes for the targeted delivery of gemcitabine to non–small cell lung cancer in vitro. J Pharm sci. 2016;105:1741–50.
Quader S, Kataoka K. Nanomaterial-enabled cancer therapy. Mol Ther. 2017;25:1501–13.
Wang H, Huang Y. Combination therapy based on nano codelivery for overcoming cancer drug resistance. Med Drug Discov. 2020;6:100024.
Alibolandi M, Ramezani M, Abnous K, Sadeghi F, Atyabi F, Asouri M, et al. In vitro and in vivo evaluation of therapy targeting epithelial-cell adhesion-molecule aptamers for non-small cell lung cancer. J Control Release. 2015;209:88–100.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–84.
Alibolandi M, Rezvani R, Farzad SA, Taghdisi SM, Abnous K, Ramezani M. Tetrac-conjugated polymersomes for integrin-targeted delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. Int J Pharm. 2017;532:581–94.
Sun W, Du Y, Liang X, Yu C, Fang J, Lu W, et al. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials. 2019;217:119264.
Bae YH, Park K. Targeted drug delivery to tumors: Myths, reality and possibility. J Control Release. 2011;153:198–205.
Acknowledgements
The authors are grateful for the Mashhad University of Medical Sciences (Grant no. 960957). This study was based on the Pharm D. thesis of SS.
Author information
Authors and Affiliations
Contributions
SS and ST: performance and formal analysis as well as writing—original draft. KA: formal analysis, review, and editing. SMT: formal analysis, review, and editing. MB: formal analysis. MR: conceptualization, supervision. MA: conceptualization, pereparation of final manuscript for submission, supervision. Finally, the paper was written through contributions of all authors. All authors have given approval to the final version of the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sanati, S., Taghavi, S., Abnous, K. et al. Fabrication of anionic dextran-coated micelles for aptamer targeted delivery of camptothecin and survivin-shRNA to colon adenocarcinoma. Gene Ther 29, 55–68 (2022). https://doi.org/10.1038/s41434-021-00234-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-021-00234-0
This article is cited by
-
The impact of anionic polymers on gene delivery: how composition and assembly help evading the toxicity-efficiency dilemma
Journal of Nanobiotechnology (2021)