Abstract
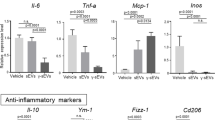
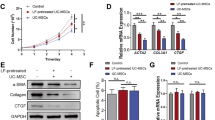
Extracellular vesicles (EVs) can mediate mesenchymal stromal cells (MSCs) paracrine effects. We aimed to evaluate the therapeutic potential of human umbilical cord perivascular cells (HUCPVCs) engineered to produce Insulin Growth Factor like-I (IGF-I) in experimental liver fibrosis and the role of EVs in this effect. HUCPVCs were engineered to produce human IGF-I (AdhIGF-I) or green fluorescence protein (AdGFP) using adenoviruses, and EVs were isolated from their conditioned medium (CM). In vitro effects of CM and EVs on hepatic stellate cells and hepatic macrophages were studied. Cells or EVs-based treatments were evaluated in thioacetamide-induced liver fibrosis in mice. The application of AdhIGF-I-HUCPVCs resulted in a further amelioration of liver fibrosis when compared to AdGFP-HUCPVCs and saline. Similarly, treatment with AdhIGF-I-HUCPVCs-derived EVs resulted in a reduction of collagen deposition and gene expression of the fibrogenic related molecules TGF-β1, α-SMA, and COL1A2. In vitro incubation of hepatic stellate cells with EVs-AdhIGF-I-HUCPVCs significantly reduced activation of fibrogenic cells. In addition, EVs-AdhIGF-I-HUCPVCs trigger hepatic macrophages to switch their phenotype towards anti-inflammatory phagocytes, which might be involved in the antifibrotic effect. Consistently, high levels of IGF-I were observed within EVs-AdhIGF-I-HUCPVCs but not in controls EVs. Our results showed that hIGF-I carrying EVs could mediate the paracrine mechanism by which AdhIGF-I-HUCPVCs reduce liver fibrosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–41.
Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18.
Bonefeld K, Moller S. Insulin-like growth factor-I and the liver. Liver Int. 2011;31:911–9.
Castilla-Cortazar I, Garcia M, Muguerza B, Quiroga J, Perez R, Santidrian S, et al. Hepatoprotective effects of insulin-like growth factor I in rats with carbon tetrachloride-induced cirrhosis. Gastroenterology. 1997;113:1682–91.
Conchillo M, de Knegt RJ, Payeras M, Quiroga J, Sangro B, Herrero JI, et al. Insulin-like growth factor I (IGF-I) replacement therapy increases albumin concentration in liver cirrhosis: results of a pilot randomized controlled clinical trial. J Hepatol. 2005;43:630–6.
Sobrevals L, Enguita M, Quiroga J, Prieto J, Fortes P. Insulin-like growth factor I (IGF-I) expressed from an AAV1 vector leads to a complete reversion of liver cirrhosis in rats. PLoS ONE. 2016;11:e0162955.
Sobrevals L, Rodriguez C, Romero-Trevejo JL, Gondi G, Monreal I, Paneda A, et al. Insulin-like growth factor I gene transfer to cirrhotic liver induces fibrolysis and reduces fibrogenesis leading to cirrhosis reversion in rats. Hepatology. 2010;51:912–21.
Ramachandran P, Iredale JP. Macrophages: central regulators of hepatic fibrogenesis and fibrosis resolution. J Hepatol. 2012;56:1417–9.
Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–6.
Fiore EJ, Dominguez LM, Bayo J, Garcia MG, Mazzolini GD. Taking advantage of the potential of mesenchymal stromal cells in liver regeneration: cells and extracellular vesicles as therapeutic strategies. World J Gastroenterol. 2018;24:2427–40.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7.
Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506.
Bayo J, Marrodan M, Aquino JB, Silva M, Garcia MG, Mazzolini G. The therapeutic potential of bone marrow-derived mesenchymal stromal cells on hepatocellular carcinoma. Liver Int. 2013;34:330–42.
Fiore EJ, Mazzolini G, Aquino JB. Mesenchymal stem/stromal cells in liver fibrosis: recent findings, old/new caveats and future perspectives. Stem Cell Rev. 2015;11:586–97.
Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12.
Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220–9.
Bayo J, Fiore E, Aquino JB, Malvicini M, Rizzo M, Peixoto E, et al. Human umbilical cord perivascular cells exhibited enhanced migration capacity towards hepatocellular carcinoma in comparison with bone marrow mesenchymal stromal cells: a role for autocrine motility factor receptor. Biomed Res Int. 2014;2014:837420.
Bayo J, Fiore E, Aquino JB, Malvicini M, Rizzo M, Peixoto E, et al. Increased migration of human mesenchymal stromal cells by autocrine motility factor (AMF) resulted in enhanced recruitment towards hepatocellular carcinoma. PLoS ONE. 2014;9:e95171.
Fiore EJ, Bayo JM, Garcia MG, Malvicini M, Lloyd R, Piccioni F, et al. Mesenchymal stromal cells engineered to produce IGF-I by recombinant adenovirus ameliorate liver fibrosis in mice. Stem Cells Dev. 2015;24:791–801.
Fiore E, Malvicini M, Bayo J, Peixoto E, Atorrasagasti C, Sierra R, et al. Involvement of hepatic macrophages in the antifibrotic effect of IGF-I-overexpressing mesenchymal stromal cells. Stem Cell Res Ther. 2016;7:172.
Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017;26:617–31.
Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79.
Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913.
Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharmaceutica Sinica B. 2016;6:287–96.
Armstrong JP, Holme MN, Stevens MM. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11:69–83.
Liu Q, Yu Z, Yuan S, Xie W, Li C, Hu Z, et al. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget. 2017;8:13048–58.
Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci USA. 2016;113:170–5.
Braid LR, Hu WG, Davies JE, Nagata LP. Engineered mesenchymal cells improve passive immune protection against lethal venezuelan equine encephalitis virus exposure. Stem Cells Transl Med. 2016;5:1026–35.
Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–54.
Chen L, Xiang B, Wang X, Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther. 2017;8:9.
Haga H, Yan IK, Takahashi K, Matsuda A, Patel T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells improve survival from lethal hepatic failure in mice. Stem Cells Transl Med. 2017;6:1262–72.
Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76.
Lou G, Yang Y, Liu F, Ye B, Chen Z, Zheng M, et al. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J Cell Mol Med. 2017;21:2963–73.
Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21:2491–502.
Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428:688–92.
Treacy O, Ryan AE, Heinzl T, O’Flynn L, Cregg M, Wilk M, et al. Adenoviral transduction of mesenchymal stem cells: in vitro responses and in vivo immune responses after cell transplantation. PLoS ONE. 2012;7:e42662.
Acknowledgements
We acknowledge Paula Rosello, Alejandrina Real, Mariana Banis, and Guillermo Gaston for technical support.
Funding
This work was supported in part by grants from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT 2015 N° 2036; PICTO 2016-0101), Fundación Allende, Fundación Alberto Roemmers, PUE0102 (CONICET), and Universidad Austral.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fiore, E., Domínguez, L.M., Bayo, J. et al. Human umbilical cord perivascular cells-derived extracellular vesicles mediate the transfer of IGF-I to the liver and ameliorate hepatic fibrogenesis in mice. Gene Ther 27, 62–73 (2020). https://doi.org/10.1038/s41434-019-0102-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-019-0102-7