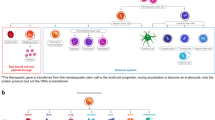
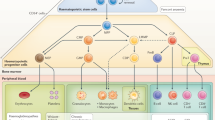
Abstract
Recent advances in genetic engineering technology and stem cell biology have spurred great interest in developing gene therapies for hereditary, as well as acquired hematological disorders. Currently, hematopoietic stem cell transplantation is used to cure disorders such as hemoglobinopathies and primary immunodeficiencies; however, this method is limited by the availability of immune-matched donors. Using autologous cells coupled with genome editing bypasses this limitation and therefore became the focus of many research groups aiming to develop efficient and safe genomic modification. Hence, gene therapy research has witnessed a noticeable growth in recent years with numerous successful achievements; however, several challenges have to be overcome before gene therapy becomes widely available for patients. In this review, I discuss tools used in gene therapy for hematological disorders, choices of target cells, and delivery vehicles with emphasis on current hurdles and attempts to solve them, and present examples of successful clinical trials to give a glimpse of current progress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, et al. T Lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science. (80-) 1995. https://doi.org/10.1126/science.270.5235.475.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006. https://doi.org/10.1016/j.cell.2006.07.024.
Gupta RM, Musunuru K. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J Clin Invest. 2014; https://doi.org/10.1172/JCI72992.
Papapetrou EP, Schambach A. Gene insertion into genomic safe harbors for human gene therapy. Mol Ther. 2016. https://doi.org/10.1038/mt.2016.38.
Maeder ML, Gersbach CA. Genome-editing technologies for gene and cell therapy. Mol Ther. 2016. https://doi.org/10.1038/mt.2016.10.
Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016. https://doi.org/10.1038/nature16526.
Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science (80-). 2016. https://doi.org/10.1126/science.aad5227.
Chen JS, Dagdas YS, Kleinstiver BP, Welch MM, Sousa AA, Harrington LB, et al. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature. 2017. https://doi.org/10.1038/nature24268.
Vakulskas CA, Dever DP, Rettig GR, Turk R, Jacobi AM, Collingwood MA, et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat Med. 2018. https://doi.org/10.1038/s41591-018-0137-0.
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016. https://doi.org/10.1038/nature17946.
Richter M, Stone D, Miao C, Humbert O, Kiem HP, Papayannopoulou T, et al. In vivo hematopoietic stem cell transduction. Hematol Oncol Clin N. Am. 2017. https://doi.org/10.1016/j.hoc.2017.06.001.
Nathwani AC, Tuddenham EGD, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-Associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011. https://doi.org/10.1056/NEJMoa1108046.
Lino CA, Harper JC, Carney JP, Timlin JA. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018. https://doi.org/10.1080/10717544.2018.1474964.
Brendel C, Goebel B, Daniela A, Brugman M, Kneissl S, Schwäble J, et al. CD133-targeted gene transfer into long-term repopulating hematopoietic stem cells. Mol Ther. 2015. https://doi.org/10.1038/mt.2014.173.
Meuwissen HJ, Gatti RA, Terasaki PI, Hong R, Good RA. Treatment of lymphopenic hypogammaglobulinemia and bone-marrow aplasia by transplantation of allogeneic marrow. N Engl J Med. 1969. https://doi.org/10.1056/NEJM196909252811302.
Hoban MD, Cost GJ, Mendel MC, Romero Z, Kaufman ML, Joglekar AV, et al. Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood. 2015. https://doi.org/10.1182/blood-2014-12-615948.
Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010. https://doi.org/10.1038/nbt.1663.
Park B, Yoo KH, Kim C. Hematopoietic stem cell expansion and generation: he ways to make a breakthrough. Blood Res. 2015. https://doi.org/10.5045/br.2015.50.4.194.
Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010. https://doi.org/10.1038/nm.2080.
de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012. https://doi.org/10.1056/NEJMoa1207285.
Ferreira MSV, Mousavi SH. Nanofiber technology in the ex vivo expansion of cord blood-derived hematopoietic stem cells. Nanomed Nanotechnol Biol Med. 2018. https://doi.org/10.1016/j.nano.2018.04.017.
Dever DP, Bak RO, Reinisch A, Camarena J, Washington G, Nicolas CE, et al. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature. 2016. https://doi.org/10.1038/nature20134.
Hanna J, Wernig M, Markoulaki S, Sun C-W, Meissner A, Cassady JP, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007. https://doi.org/10.1126/science.1152092.
Wahlster L, Daley GQ. Progress towards generation of human haematopoietic stem cells. Nat Cell Biol. 2016. https://doi.org/10.1038/ncb3419.
Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013. https://doi.org/10.1038/nbt.2678.
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science (80-.). 2018. https://doi.org/10.1126/science.aar6711.
Batta K, Florkowska M, Kouskoff V, Lacaud G. Direct Reprogramming of murine fibroblasts to hematopoietic progenitor cells. Cell Rep. 2014. https://doi.org/10.1016/j.celrep.2014.11.002.
Sandler VM, Lis R, Liu Y, Kedem A, James D, Elemento O, et al. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature. 2014. https://doi.org/10.1038/nature13547.
Milone MC, O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018. https://doi.org/10.1038/s41375-018-0106-0.
Yu K-R, Natanson H, Dunbar CE. Gene editing of human hematopoietic stem and progenitor cells: promise and potential hurdles. Hum Gene Ther. 2016. https://doi.org/10.1089/hum.2016.107.
VandenDriessche T, Chuah MK. Hemophilia gene therapy: ready for prime time? Hum Gene Ther. 2017. https://doi.org/10.1089/hum.2017.116.
Naso MF, Tomkowicz B, Perry WL, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017. https://doi.org/10.1007/s40259-017-0234-5.
Liang X, Potter J, Kumar S, Zou Y, Quintanilla R, Sridharan M, et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J Biotechnol. 2015. https://doi.org/10.1016/j.jbiotec.2015.04.024.
De Ravin SS, Reik A, Liu P-Q, Li L, Wu X, Su L, et al. Targeted gene addition in human CD34(+) hematopoietic cells for correction of X-linked chronic granulomatous disease. Nat Biotechnol. 2016;34:424–9.
Pestina TI, Hargrove PW, Jay D, Gray JT, Boyd KM, Persons DA. Correction of murine sickle cell disease using gamma-globin lentiviral vectors to mediate high-level expression of fetal hemoglobin. Mol Ther. 2009. mt2008259 [pii]/r10.1038/mt.2008.259.
Chang KH, Smith SE, Sullivan T, Chen K, Zhou Q, West JA, et al. Long-Term engraftment and fetal globin induction upon BCL11A gene editing in bone-marrow-derived CD34+ hematopoietic stem and progenitor cells. Mol Ther. 2017. https://doi.org/10.1016/j.omtm.2016.12.009.
Ribeil J-A, Hacein-Bey-Abina S, Payen E, Magnani A, Semeraro M, Magrin E, et al. Gene therapy in a patient with sickle cell disease. N Engl J Med. 2017. https://doi.org/10.1056/NEJMoa1609677.
Jayavaradhan R, Malik P. Genetic therapies for sickle cell disease. Pediatr Clin N Am. 2018;65:465–80.
Monahan PE, Sun J, Gui T, Hu G, Hannah WB, Wichlan DG, et al. Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial. Hum Gene Ther. 2015. https://doi.org/10.1089/hum.2014.106.
Wang CX, Cannon PM. The clinical applications of genome editing in HIV. Blood. 2016. https://doi.org/10.1182/blood-2016-01-678144.
Maier DA, Brennan AL, Jiang S, Binder-Scholl GK, Lee G, Plesa G, et al. Efficient clinical scale gene modification via zinc finger nuclease-targeted disruption of the HIV co-receptor CCR5. Hum Gene Ther. 2013. https://doi.org/10.1089/hum.2012.172.
Ebina H, Misawa N, Kanemura Y, Koyanagi Y. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep. 2013. https://doi.org/10.1038/srep02510.
Huang Z, Tomitaka A, Raymond A, Nair M. Current application of CRISPR/Cas9 gene-editing technique to eradication of HIV/AIDS. Gene Ther. 2017. https://doi.org/10.1038/gt.2017.35.
Schumann K, Lin S, Boyer E, Simeonov DR, Subramaniam M, Gate RE, et al. Generation of knock-in primary human T cells using. Proc Natl Acad Sci USA. 2015. https://doi.org/10.1073/pnas.1512503112.
de Lima M, McMannis J, Gee A, Komanduri K, Couriel D, Andersson BS, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant. 2008. https://doi.org/10.1038/sj.bmt.1705979.
Rizo A, Dontje B, Vellenga E, De Haan G, Sehuringa JJ. Long-term maintenance of human hematopoietic stem/progenitor cells by expression of BMI1. Blood. 2008. https://doi.org/10.1182/blood-2007-08-106666.
Nishino T, Miyaji K, Ishiwata N, Arai K, Yui M, Asai Y, et al. Ex vivo expansion of human hematopoietic stem cells by a small-molecule agonist of c-MPL. Exp Hematol. 2009. https://doi.org/10.1016/j.exphem.2009.09.001.
Csaszar E, Kirouac DC, Yu M, Wang W, Qiao W, Cooke MP, et al. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell. 2012. https://doi.org/10.1016/j.stem.2012.01.003.
Cutler C, Multani P, Robbins D, Kim HT, Le T, Hoggatt J, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013. https://doi.org/10.1182/blood-2013-05-503177.
Fares I, Chagraoui J, Gareau Y, Gingras S, Ruel R, Mayotte N, et al. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science (80-). 2014. https://doi.org/10.1126/science.1256337.
Horwitz ME, Chao NJ, Rizzieri DA, Long GD, Sullivan KM, Gasparetto C, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014. https://doi.org/10.1172/JCI74556.
Chaurasia P, Gajzer DC, Schaniel C, Souza SD, Hoffman R, D’Souza S, et al. Epigenetic reprogramming induces the expansion of cord blood stem cells. J Clin Invest. 2014. https://doi.org/10.1172/JCI70313.2378.
Xie X-Q, Yang P, Zhang Y, Zhang P, Wang L, Ding Y, et al. Discovery of novel INK4C small-molecule inhibitors to promote human and murine hematopoietic stem cell ex vivo expansion. Sci Rep. 2015. https://doi.org/10.1038/srep18115.
Wagner JE, Brunstein CG, Boitano AE, Defor TE, McKenna D, Sumstad D, et al. Phase I/II trial of StemRegenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell. 2016. https://doi.org/10.1016/j.stem.2015.10.004.
Shen B, Zhang Y, Dai W, Ma Y, Jiang Y, Aguila J, et al. Ex-vivo expansion of nonhuman primate CD34+ cells by stem cell factor Sall4B. Stem Cell Res Ther. 2016. https://doi.org/10.1186/s13287-016-0413-1.
Wang L, Guan X, Wang H, Shen B, Zhang Y, Ren Z, et al. A small-molecule/cytokine combination enhances hematopoietic stem cell proliferation via inhibition of cell differentiation. Stem Cell Res Ther. 2017. https://doi.org/10.1186/s13287-017-0625-z.
Li Z, Qian P, Shao W, Shi H, He XC, Gogol M, et al. Suppression of m6A reader Ythdf2 promotes hematopoietic stem cell expansion. Cell Res. 2018. https://doi.org/10.1038/s41422-018-0072-0.
Zhao K, Zheng WW, Dong XM, Yin RH, Gao R, Li X, et al. EDAG promotes the expansion and survival of human CD34+cells. PLoS ONE. 2018. https://doi.org/10.1371/journal.pone.0190794.
Guo B, Huang X, Lee MR, Lee SA, Broxmeyer HE. Antagonism of PPAR-γ signaling expands human hematopoietic stem and progenitor cells by enhancing glycolysis. Nat Med. 2018. https://doi.org/10.1038/nm.4477.
Ledran MH, Krassowska A, Armstrong L, Dimmick I, Renström J, Lang R, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008. https://doi.org/10.1016/j.stem.2008.06.001.
Woods N-B, Parker AS, Moraghebi R, Lutz MK, Firth AL, Brennand KJ, et al. Brief report: efficient generation of hematopoietic precursors and progenitors from human pluripotent stem cell lines. Stem Cells. 2011. https://doi.org/10.1002/stem.657.
Real PJ, Ligero G, Ayllon V, Ramos-Mejia V, Bueno C, Gutierrez-Aranda I, et al. SCL/TAL1 regulates hematopoietic specification from human embryonic stem cells. Mol Ther. 2012 https://doi.org/10.1038/mt.2012.49.
Kennedy M, Awong G, Sturgeon CM, Ditadi A, LaMotte-Mohs R, Zúñiga-Pflücker JC, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012. https://doi.org/10.1016/j.celrep.2012.11.003.
Park TS, Zimmerlin L, Zambidis ET. Efficient and simultaneous generation of hematopoietic and vascular progenitors from human induced pluripotent stem cells. Cytom Part A. 2013. https://doi.org/10.1002/cyto.a.22090.
Doulatov S, Vo LT, Chou SS, Kim PG, Arora N, Li H, et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell. 2013. https://doi.org/10.1016/j.stem.2013.09.002.
Ran D, Shia WJ, Lo MC, Fan JB, Knorr DA, Ferrell PI, et al. RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood. 2013. https://doi.org/10.1182/blood-2012-08-451641.
Amabile G, Welner RS, Nombela-Arrieta C, D’Alise AM, Di Ruscio A, Ebralidze AK, et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013. https://doi.org/10.1182/blood-2012-06-434407.
Suzuki N, Yamazaki S, Yamaguchi T, Okabe M, Masaki H, Takaki S, et al. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Ther. 2013. https://doi.org/10.1038/mt.2013.71.
Ramos-Mejía V, Navarro-Montero O, Ayllón V, Bueno C, Romero T, Real PJ, et al. HOXA9 promotes hematopoietic commitment of human embryonic stem cells. Blood. 2014. https://doi.org/10.1182/blood-2014-03-558825.
Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat Biotechnol. 2014. https://doi.org/10.1038/nbt.2915.
Gori JL, Butler JM, Chan YY, Chandrasekaran D, Poulos MG, Ginsberg M, et al. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J Clin Invest. 2015. https://doi.org/10.1172/JCI79328.
Jackson M, Ma R, Taylor AH, Axton RA, Easterbrook J, Kydonaki M, et al. Enforced expression of HOXB4 in human embryonic stem cells enhances the production of hematopoietic progenitors but has no effect on the maturation of red blood cells. Stem Cells Transl Med. 2016. https://doi.org/10.5966/sctm.2015-0324.
Saxena S, Rönn RE, Guibentif C, Moraghebi R, Woods NB. Cyclic AMP signaling through Epac axis modulates human hemogenic endothelium and enhances hematopoietic cell generation. Stem Cell Rep. 2016;6:692–703.
Li X, Xia C, Wang T, Liu L, Zhao Q, Yang D, et al. Pyrimidoindole derivative UM171 enhances derivation of hematopoietic progenitor cells from human pluripotent stem cells. Stem Cell Res. 2017;21:32–39.
Sugimura R, Jha DK, Han A, Soria-Valles C, Da Rocha EL, Lu YF, et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature. 2017. https://doi.org/10.1038/nature22370.
Duan F, Huang R, Zhang F, Zhu Y, Wang L, Chen X, et al. Biphasic modulation of insulin signaling enables highly efficient hematopoietic differentiation from human pluripotent stem cells. Stem Cell Res Ther. 2018. https://doi.org/10.1186/s13287-018-0934-x.
Tan Y-T, Ye L, Xie F, Beyer AI, Muench MO, Wang J, et al. Respecifying human iPSC-derived blood cells into highly engraftable hematopoietic stem and progenitor cells with a single factor. Proc Natl Acad Sci USA. 2018. https://doi.org/10.1073/pnas.1718446115.
Acknowledgements
I would like to thank Zahraa Al-Saif for her help in producing the illustrations in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that he has no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Saif, A.M. Gene therapy of hematological disorders: current challenges. Gene Ther 26, 296–307 (2019). https://doi.org/10.1038/s41434-019-0093-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-019-0093-4