Abstract
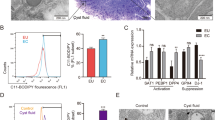
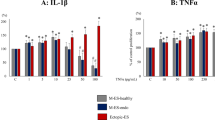
Development of gene therapy for endometriosis requires inhibition of vascularization in endometrial lesions. We have previously developed CXCR4 receptor-targeted siRNA carrier L1 and observed efficient RNAi-mediated down-regulation of VEGFA gene expression in endothelial cells followed by decrease in VEGFA protein production and inhibition of cell migration. In this study we evaluated L1 carrier as non-viral vector for anti-VEGFA siRNA delivery into endometrial implants in rat subcutaneous endometriosis model created by subcutaneous auto-transplantation of uterus horn’s fragments. Therapeutic anti-angiogenic efficiency of anti-VEGFA siRNA/L1 polyplexes was evaluated by lesion size measurement, histopathologic examination, immunohistochemical staining and real-time reverse transcriptase-PCR analysis. After in vivo administration of anti-VEGFA siRNA we observed a 55–60% inhibition of endometriotic lesions growth and approximately two-fold decrease in VEGFA gene expression in comparison with untreated implants. Results of immunohistochemical examination of endometriotic lesions confirmed anti-angiogenic effects of anti-VEGFA siRNA/L1 polyplexes. Ultimately, our results demonstrate the efficiency of anti-angiogenic treatment of EM by means of anti-VEGFA siRNA delivery with L1 peptide-based carrier.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Baranov VS, Ivaschenko TE, Liehr T, Yarmolinskaya MI. Systems genetics view of endometriosis: a common complex disorder. Eur J Obstet Gynecol Reprod Biol. 2015;185:59–65.
Oosterlynck DJ, Meuleman C, Sobis H, Vandeputte M, Koninckx PR. Angiogenic activity of peritoneal fluid from women with endometriosis. Fertil Steril. 1993;59:778–82.
Shubina AN, Egorova AA, Baranov VS, Kiselev AV. Recent advances in gene therapy of endometriosis. Recent Pat DNA Gene Seq. 2013;7:169–78.
Kyama CM, Debrock S, Mwenda JM, D’Hooghe TM. Potential involvement of the immune system in the development of endometriosis. Reprod Biol Endocrinol. 2003;1:123.
Buchweitz O, Staebler A, Wülfing P, Hauzman E, Greb R, Kiesel L. COX-2 overexpression in peritoneal lesions is correlated with nonmenstrual chronic pelvic pain. Eur J Obstet Gynecol Reprod Biol. 2006;124:216–21.
Hornung D. Chemokine bioactivity of RANTES in endometriotic and normal endometrial stromal cells and peritoneal fluid. Mol Hum Reprod. 2001;7:163–8.
McLaren J, Prentice A, Charnock-Jones DS, Smith SK. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod. 1996;11:220–3.
González-Ramos R, Donnez J, Defrère S, Leclercq I, Squifflet J, Lousse JC, et al. Nuclear factor-kappa B is constitutively activated in peritoneal endometriosis. Mol Hum Reprod. 2007;13:503–9.
Pauli SA, Tang H, Wang J, Bohlen P, Posser R, Hartman T, et al. The vascular endothelial growth factor (VEGF)/VEGF receptor 2 pathway is critical for blood vessel survival in corpora lutea of pregnancy in the rodent. Endocrinology. 2005;146:1301–11.
Dabrosin C, Gyorffy S, Margetts P, Ross C, Gauldie J. Therapeutic effect of angiostatin gene transfer in a murine model of endometriosis. Am J Pathol. 2002;161:909–18.
Zhao MD, Sun YM, Fu GF, Du YZ, Chen FY, Yuan H, et al. Gene therapy of endometriosis introduced by polymeric micelles with glycolipid-like structure. Biomaterials. 2012;33:634–43.
Sun Y, Che X, Zhu L, Zhao M, Fu G, Huang X, et al. Pigment epithelium derived factor inhibits the growth of human endometrial implants in nude mice and of ovarian endometriotic stromal cells in vitro. PLoS One. 2012;7:e45223.
Wang N, Liu B, Liang L, Wu Y, Xie H, Huang J, et al. Antiangiogenesis therapy of endometriosis using PAMAM as a gene vector in a noninvasive animal model. Biomed Res Int. 2014;2014:546479 https://doi.org/10.1155/2014/546479.
Zhao MD, Cheng JL, Yan JJ, Chen FY, Sheng JZ, Sun DL, et al. Hyaluronic acid reagent functional chitosan-PEI conjugate with AQP2-siRNA suppressed endometriotic lesion formation. Int J Nanomed. 2016;11:1323–36.
Rein DT, Schmidt T, Bauerschmitz G, Hampl M, Beyer IM, Paupoo AAV, et al. Treatment of endometriosis with a VEGF-targeted conditionally replicative adenovirus. Fertil Steril. 2010;93:2687–94.
Paupoo AAV, Zhu ZB, Wang M, Rein DT, Starzinski-Powitz A, Curiel DT. A conditionally replicative adenovirus, CRAd-S-pK7, can target endometriosis with a cell-killing effect. Hum Reprod. 2010;25:2068–83.
Koippallil Gopalakrishnan AR, Pandit H, Metkari SM, Warty N, Madan T. Adenoviral vector encoding soluble Flt-1 engineered human endometrial mesenchymal stem cells effectively regress endometriotic lesions in NOD/SCID mice. Gene Ther. 2016;23:580–91.
Wickham TJ. Ligand-directed targeting of genes to the site of disease. Nat Med. 2003;9:135–9.
Juarez J, Bendall L, Bradstock K. Chemokines and their receptors as therapeutic targets: the role of the SDF-1 / CXCR4 axis. Curr Pharm Des. 2004;10:1245–59.
Andre F, Soria JC, Assi H, Delaloge S, Spielmann M. Expression of chemokine receptors by cancer cells. Bull Cancer. 2004;91:S254–6.
Driessen WHP, Fujii N, Tamamura H, Sullivan SM. Development of peptide-targeted lipoplexes to CXCR4-expressing rat glioma cells and rat proliferating endothelial cells. Mol Ther. 2008;16:516–24.
Salcedo R, Oppenheim JJ. Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation. 2003;10:359–70.
Flores I, Rivera E, Ruiz LA, Santiago OI, Vernon MW, Appleyard CB. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease. Fertil Steril. 2007;87:1180–99.
Ruiz A, Salvo VA, Ruiz LA, Báez P, García M, Flores I. Basal and steroid hormone-regulated expression of CXCR4 in human endometrium and endometriosis. Reprod Sci. 2010;17:894–903.
Leconte M, Chouzenoux S, Nicco C, Chéreau C, Arkwright S, Santulli P, et al. Role of the CXCL12-CXCR4 axis in the development of deep rectal endometriosis. J Reprod Immunol. 2014;103:45–52.
Egorova A, Kiselev A, Hakli M, Ruponen M, Baranov V, Urtti A. Chemokine-derived peptides as carriers for gene delivery to CXCR4 expressing cells. J Gene Med. 2009;11:772–81.
Egorova A, Bogacheva M, Shubina A, Baranov V, Kiselev A. Development of a receptor-targeted gene delivery system using CXCR4 ligand-conjugated cross-linking peptides. J Gene Med. 2014;16:336–51.
Kiselev A, Egorova A, Laukkanen A, Baranov V, Urtti A. Characterization of reducible peptide oligomers as carriers for gene delivery. Int J Pharm. 2013;441:736–47.
Egorova A, Shubina A, Sokolov D, Selkov S, Baranov V, Kiselev A. CXCR4-targeted modular peptide carriers for efficient anti-VEGF siRNA delivery. Int J Pharm. 2016;515:431–40.
Fu G, Che X, Sun Y, Huang X, Xu H, Zhou C, et al. Pigment epithelial-derived factor expression in endometriotic lesions in a rat model of endometriosis. Acta Histochem. 2013;115:301–7.
Pelch KE, Sharpe-Timms KL, Nagel SC. Mouse model of surgically-induced endometriosis by auto-transplantation of uterine tissue. J Vis Exp. 2012;59:3396 https://doi.org/10.3791/3396.
Grümmer R. Animal models in endometriosis research. Hum Reprod Update. 2006;12:641–9.
Ricci AG, Olivares CN, Bilotas MA, Meresman GF, Barañao RI. Effect of vascular endothelial growth factor inhibition on endometrial implant development in a murine model of endometriosis. Reprod Sci. 2011;18:614–22.
Asante A, Taylor RN. Endometriosis: the role of neuroangiogenesis. Annu Rev Physiol. 2011;73:163–82.
Mokhtarzadeh A, Alibakhshi A, Hashemi M, Hejazi M, Hosseini V, de la Guardia M, Ramezani M. Biodegradable nano-polymers as delivery vehicles for therapeutic small non-coding ribonucleic acids. J Control Release. 2017;245:116–26.
Egorova AA, Kiselev AV. Peptide modules for overcoming barriers of nucleic acids transport to cells. Curr Top Med Chem. 2016;16:330–42.
De Fougerolles A, Frank-Kamenetsky M, Manoharan M, Rajeev KG, Hadwiger P. IRNA agents targeting VEGF. U.S. Patent No. 7,919,473. Washington, DC: U.S. Patent and Trademark Office; 2011.
Xu H, Jiang B, Meng L, Ren T, Zeng Y, Wu J, et al. N-α-acetyltransferase 10 protein inhibits apoptosis through RelA/p65-regulated MCL1 expression. Carcinogenesis. 2012;33:1193–202.
Acknowledgements
We are thankful to Tatyana Kleimenova for technical assistance and Sara Gjurgji for English correction of the article. This work was supported by Russian Science Foundation grant 14-15-00737. Also we acknowledge partial financial support of peptide synthesis and antibody expenses by Russian Foundation for Basic Research grants 15-04-00591 and 18-015-00357. Marianna Maretina is supported by President of Russian Federation scholarship (SP-822.2018.4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Egorova, A., Petrosyan, M., Maretina, M. et al. Anti-angiogenic treatment of endometriosis via anti-VEGFA siRNA delivery by means of peptide-based carrier in a rat subcutaneous model. Gene Ther 25, 548–555 (2018). https://doi.org/10.1038/s41434-018-0042-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-018-0042-7
This article is cited by
-
Delivery technologies for women’s health applications
Nature Reviews Bioengineering (2023)
-
Single-cell analysis of endometriosis reveals a coordinated transcriptional programme driving immunotolerance and angiogenesis across eutopic and ectopic tissues
Nature Cell Biology (2022)