Abstract
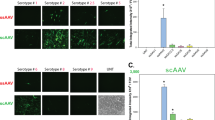
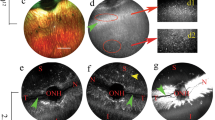
AAV gene therapy approaches in the posterior eye resulted in the first FDA-approved gene therapy-based drug. However, application of AAV vectorology to the anterior eye has yet to enter even a Phase I trial. Furthermore, the simple and safe subconjunctival injection has been relatively unexplored in regard to AAV vector transduction. To determine the utility of this route for the treatment of various ocular disorders, a survey of gene delivery via natural AAV serotypes was performed and correlated to reported cellular attachment factors. AAV serotypes packaged with a self-complementary reporter were administered via subconjunctival injection to WT mice. Subconjunctival injection of AAV vectors was without incidence; however, vector shedding in tears was noted weeks following administration. AAV transduction was serotype dependent in anterior segment tissues including the eye lid, conjunctiva, and cornea, as well as the periocular tissues including muscle. Transgene product in the cornea was highest for AAV6 and AAV8, however, their corneal restriction was remarkably different; AAV6 appeared restricted to the endothelium layer while AAV8 efficiently transduced the stromal layer. Reported AAV cellular receptors were not well correlated to vector transduction; although, in some cases they were conserved among mouse and human ocular tissues. Subconjunctival administration of particular AAV serotypes may be a simple and safe targeted gene delivery route for ocular surface, muscular, corneal, and optic nerve diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9.
Feuer WJ, Schiffman JC, Davis JL, Porciatti V, Gonzalez P, Koilkonda RD, et al. Gene therapy for Leber hereditary optic neuropathy: initial results. Ophthalmology. 2016;123:558–70.
Chong RS, Su DH, Tsai A, Jiang Y, Htoon HM, Lamoureux EL, et al. Patient acceptance and attitude toward an alternative method of subconjunctival injection for the medical treatment of glaucoma. J Glaucoma. 2013;22:190–4.
Salganik M, Hirsch ML, Samulski RJ. Adeno-associated virus as a mammalian DNA vector. Microbiol Spectr. 2015;3:1–32.
Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–6.
Atchison RW, Casto BC, Hammon WM. Electron microscopy of adenovirus-associated virus (AAV) in cell cultures. Virology. 1966;29:353–7.
Samulski RJ, Srivastava A, Berns KI, Muzyczka N. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell. 1983;33:135–43.
Srivastava A, Lusby EW, Berns KI. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983;45:555–64.
Chaudhary K, Moore H, Tandon A, Gupta S, Khanna R, Mohan RR. Nanotechnology and adeno-associated virus-based decorin gene therapy ameliorates peritoneal fibrosis. Am J Physiol Renal Physiol. 2014;307:F777–82.
Igarashi T, Miyake K, Suzuki N, Kato K, Takahashi H, Ohara K, et al. New strategy for in vivo transgene expression in corneal epithelial progenitor cells. Curr Eye Res. 2002;24:46–50.
Mohan RR, Schultz GS, Hong JW, Wilson SE. Gene transfer into rabbit keratocytes using AAV and lipid-mediated plasmid DNA vectors with a lamellar flap for stromal access. Exp Eye Res. 2003;76:373–83.
Mohan RR, Sharma A, Netto MV, Sinha S, Wilson SE. Gene therapy in the cornea. Prog Retin Eye Res. 2005;24:537–59.
Sharma A, Tovey JC, Ghosh A, Mohan RR. AAV serotype influences gene transfer in corneal stroma in vivo. Exp Eye Res. 2010;91:440–8.
Mohan RR, Sharma A, Cebulko TC, Tandon A. Vector delivery technique affects gene transfer in the cornea in vivo. Mol Vis. 2010;16:2494–501.
Mohan RR, Tovey JC, Sharma A, Schultz GS, Cowden JW, Tandon A. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS ONE. 2011;6:e26432.
Mohan RR, Tandon A, Sharma A, Cowden JW, Tovey JC. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Invest Ophthalmol Vis Sci. 2011;52:4833–41.
Mohan RR, Sinha S, Tandon A, Gupta R, Tovey JC, Sharma A. Efficacious and safe tissue-selective controlled gene therapy approaches for the cornea. PLoS ONE. 2011;6:e18771.
Vance M, Llanga T, Bennett W, Woodard K, Murlidharan G, Chungfat N, et al. AAV gene therapy for MPS1-associated corneal blindness. Sci Rep. 2016;6:22131.
Hippert C, Ibanes S, Serratrice N, Court F, Malecaze F, Kremer EJ, et al. Corneal transduction by intra-stromal injection of AAV vectors in vivo in the mouse and ex vivo in human explants. PLoS ONE. 2012;7:e35318.
Tsai ML, Chen SL, Chou PI, Wen LY, Tsai RJ, Tsao YP. Inducible adeno-associated virus vector-delivered transgene expression in corneal endothelium. Invest Ophthalmol Vis Sci. 2002;43:751–7.
Bogner B, Boye SL, Min SH, Peterson JJ, Ruan Q, Zhang Z, et al. Capsid mutated adeno-associated virus delivered to the anterior chamber results in efficient transduction of trabecular meshwork in mouse and rat. PLoS ONE. 2015;10:e0128759.
Buie LK, Rasmussen CA, Porterfield EC, Ramgolam VS, Choi VW, Markovic-Plese S, et al. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest Ophthalmol Vis Sci. 2010;51:236–48.
O’Callaghan J, Crosbie DE, Cassidy PS, Sherwood JM, Flügel-Koch C, Lütjen-Drecoll E, et al. Therapeutic potential of AAV-mediated MMP-3 secretion from corneal endothelium in treating glaucoma. Hum Mol Genet. 2017;26:1230–1246.
Wang L, Xiao R, Andres-Mateos E, Vandenberghe LH. Single stranded adeno-associated virus achieves efficient gene transfer to anterior segment in the mouse eye. PLoS ONE. 2017;12:e0182473.
Thomas PB, Samant DM, Selvam S, Wei RH, Wang Y, Stevenson D, et al. Adeno-associated virus-mediated IL-10 gene transfer suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis. Invest Ophthalmol Vis Sci. 2010;51:5137–44.
Cheng HC, Yeh SI, Tsao YP, Kuo PC. Subconjunctival injection of recombinant AAV-angiostatin ameliorates alkali burn induced corneal angiogenesis. Mol Vis. 2007;13:2344–52.
Igarashi T, Miyake K, Asakawa N, Miyake N, Shimada T, Takahashi H. Direct comparison of administration routes for AAV8-mediated ocular gene therapy. Curr Eye Res. 2013;38:569–77.
Lai LJ, Xiao X, Wu JH. Inhibition of corneal neovascularization with endostatin delivered by adeno-associated viral (AAV) vector in a mouse corneal injury model. J Biomed Sci. 2007;14:313–22.
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. New Engl J Med. 2011;365:2357–65.
Nathwani AC, Nienhuis AW, Davidoff AM. Our journey to successful gene therapy for hemophilia B. Hum Gene Ther. 2014;25:923–6.
Szybalski W. The 50th anniversary of gene therapy: beginnings and present realities. Gene. 2013;525:151–4.
Schwartz AE, Rodrigues MM, Brown K, Gaskins R, Hackett J, Thomas G, et al. Corneal opacification in C57BL/6J mice. Cornea. 1982;1:195–204.
Koehn D, Meyer KJ, Syed NA, Anderson MG. Ketamine/xylazine-induced corneal damage in mice. PLoS ONE. 2015;10:e0132804.
Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. New Engl J Med. 2014;371:1994–2004.
Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–45.
Qiu J, Mizukami H, Brown KE. Adeno-associated virus 2 co-receptors? Nat Med. 1999;5:467–8.
Weller ML, Amornphimoltham P, Schmidt M, Wilson PA, Gutkind JS, Chiorini JA. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nat Med. 2010;16:662–4.
Pillay S, Meyer NL, Puschnik AS, Davulcu O, Diep J, Ishikawa Y, et al. An essential receptor for adeno-associated virus infection. Nature. 2016;530:108–12.
Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80:9831–6.
Mietzsch M, Broecker F, Reinhardt A, Seeberger PH, Heilbronn R. Differential adeno-associated virus serotype-specific interaction patterns with synthetic heparins and other glycans. J Virol. 2014;88:2991–3003.
Wu Z, Miller E, Agbandje-McKenna M, Samulski RJ. Alpha2,3 andalpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol. 2006;80:9093–103.
Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001;75:6884–93.
Rocha EM, Di Pasquale G, Riveros PP, Quinn K, Handelman B, Chiorini JA. Transduction, tropism, and biodistribution of AAV vectors in the lacrimal gland. Invest Ophthalmol Vis Sci. 2011;52:9567–72.
Li SK, Hao J, Liu H, Lee JH. MRI study of subconjunctival and intravitreal injections. J Pharm Sci. 2012;101:2353–63.
Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12:348–60.
Hewitt FC, Li C, Gray SJ, Cockrell S, Washburn M, Samulski RJ. Reducing the risk of adeno-associated virus (AAV) vector mobilization with AAV type 5 vectors. J Virol. 2009;83:3919–29.
Li C, Goudy K, Hirsch M, Asokan A, Fan Y, Alexander J, et al. Cellular immune response to cryptic epitopes during therapeutic gene transfer. Proc Natl Acad Sci USA. 2009;106:10770–4.
Li C, Hirsch M, DiPrimio N, Asokan A, Goudy K, Tisch R, et al. Cytotoxic-T-lymphocyte-mediated elimination of target cells transduced with engineered adeno-associated virus type 2 vector in vivo. J Virol. 2009;83:6817–24.
Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4:120ra15.
Li Q, Miller R, Han PY, Pang J, Dinculescu A, Chiodo V, et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis. 2008;14:1760–9.
Amado D, Mingozzi F, Hui D, Bennicelli JL, Wei Z, Chen Y, et al. Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci Transl Med. 2010;2:21ra16.
Gilger BC. Immune relevant models for ocular inflammatory diseases. ILAR J. 2018:1–11.
Hirsch ML, Conatser LM, Smith SM, Salmon JH, Wu J, Buglak NE, et al. AAV vector-meditated expression of HLA-G reduces injury-induced corneal vascularization, immune cell infiltration, and fibrosis. Sci Rep. 2017;7:17840.
Song L, Li X, Jayandharan GR, Wang Y, Aslanidi GV, Ling C, et al. High-efficiency transduction of primary human hematopoietic stem cells and erythroid lineage-restricted expression by optimized AAV6 serotype vectors in vitro and in a murine xenograft model in vivo. PLoS ONE. 2013;8:e58757.
Song L, Kauss MA, Kopin E, Chandra M, Ul-Hasan T, Miller E, et al. Optimizing the transduction efficiency of capsid-modified AAV6 serotype vectors in primary human hematopoietic stem cells in vitro and in a xenograft mouse model in vivo. Cytotherapy. 2013;15:986–98.
Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–28.
Llanga T, Nagy N, Conatser L, Dial C, Sutton RB, Hirsch ML. Structure-based designed nano-dysferlin significantly improves dysferlinopathy in BLA/J mice. Mol Ther. 2017;25:2150–2162.
Ye L, Yu H, Li C, Hirsch ML, Zhang L, Samulski RJ, et al. Adeno-associated virus vector mediated delivery of the HBV genome induces chronic hepatitis B virus infection and liver fibrosis in mice. PLoS ONE. 2015;10:e0130052.
Acknowledgements
This study was supported by grants from the NIH RO1AI072176-06A1 (MH), RO1AR064369-01A1 (MH), and Education Bureau of Hunan Province 14B112 (LJS). A portion of the imaging was done using the Neuroscience Center Microscopy Core Facility equipment, which is supported by funding from the NIH-NINDS Neuroscience Center Support Grant P30 NS045892 and the NIH-NICHD Intellectual and Developmental Disabilities Research Center Support Grant U54HD079124. The authors thank the Vector Core at the University of North Carolina for providing the AAV vectors used in this study, the CGIBD Histology Core and histology technician, Carolyn Suitt, for the work of tissue processing and sectioning, the Animal Histopathology and laboratory Medicine Core and Dr. Ling Wang for the clinical services, the Microscopy Core Facility of the Neuroscience Center and Dr. Michelle S. Itano for the valuable technical assistance in confocal imaging, Dr. Hua Mei for reviewing the data, and Jerry Wu for manuscript proofreading.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Matthew Hirsch has several licensed patents not related to this report and has received royalties from Fortress Biotech and Asklepios BioPharmaceutical. Matthew Hirsch is a consultant to Tamid Bio.
Electronic supplementary material
41434_2018_35_MOESM2_ESM.tif
Figure S1:Vector Characterization. (A) Viral genome integrity assessment by alkaline electrophoresis followed by SYBR Gold staining. (B) Vector purity examination by silver staining of AAV capsid proteins.
41434_2018_35_MOESM3_ESM.tif
Figure S2: Safety analysis. (A) Changes in body weight during the experiment; (B) Comparison of activity of ALT in serum obtained pre-, 2 weeks and 8 weeks post-injection. (C) Quantitative evaluation of clinical histopathology scores of H&E stained sections.
41434_2018_35_MOESM4_ESM.jpg
Figure S3: Southern blotting detection of mouse ß-actin in tears. Tear samples collected at 1 week (upper panel) and 4 week (lower panel) post-subconjunctival injection were subjected to two rounds of PCR using mouse ß-actin primer set and detected by mouse ß-actin specific probe via Southern blotting. Viral vector genome plus host cell gDNA was used as positive control template.
41434_2018_35_MOESM5_ESM.jpg
Figure S4: AAV receptor analysis in mouse conjunctiva (A) and retina (B) by immunofluorescence staining. Anti-HS antibody, anti-EGFR antibody, anti-67 KDa Lam R antibody (recognizes both 37KDa Lam R precursor and 67KDa Lam R) and anti-AAVR antibody were used for HSPG, EGFR, 37/67 KDa Lam R and AAVR, respectively. WGA, SNA and MAL I were used for staining of multivalent sialic acid, α2, 6 sialic acid and α2, 3 sialic acid, respectively. NC-1: Negative controls (no primary antibody) for EGFR, 37/67 KDa Lam R and AAVR staining; NC-2: Negative controls (no primary antibody) for HSPG staining; NC-3: Negative controls for sialic acid staining. Scale Bar=20 µm.
41434_2018_35_MOESM6_ESM.jpg
Figure S5: Representative negative control images. Negative controls for WGA staining in Fig. 5 (a, b, c & d). Negative controls for SNA staining in Fig. 5 (e, f, g & h). Negative controls (no primary antibody) for AAVR staining in Fig. 5 (i & k). Negative controls (no primary antibody) for longer exposure time used for the stromal layer of Lam R and AAVR staining (j). Negative controls (no primary antibody) for EGFR and 37/67 KDa Lam R staining in Fig. 5, (l, m, n & o). Negative controls for MAL I staining in Fig. 5 (p, q, r & s).
Rights and permissions
About this article
Cite this article
Song, L., Llanga, T., Conatser, L.M. et al. Serotype survey of AAV gene delivery via subconjunctival injection in mice. Gene Ther 25, 402–414 (2018). https://doi.org/10.1038/s41434-018-0035-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-018-0035-6
This article is cited by
-
Multiplex viral tropism assay in complex cell populations with single-cell resolution
Gene Therapy (2022)
-
Effect of connective tissue growth factor gene editing using adeno-associated virus–mediated CRISPR–Cas9 on rabbit glaucoma filtering surgery outcomes
Gene Therapy (2021)
-
A 2020 vision of ocular gene therapy
Gene Therapy (2021)
-
Emerging Technologies to Solve the Key Issues in Endothelial Keratoplasty
Current Ophthalmology Reports (2020)
-
AAV-mediated expression of HLA-G1/5 reduces severity of experimental autoimmune uveitis
Scientific Reports (2019)