Abstract
Objectives
To provide evidence for long-term outcomes for margin-controlled excision of eyelid melanoma.
Methods
Retrospective single-centre observational case series of patients treated for eyelid melanoma between 2007 and 2016, with a minimum of 5-year follow-up. Tumour excision involved rush-paraffin en face horizontal sections and delayed repair (Slow Mohs; SM).
Results
Twenty-two cases were seen with a survival of 91% (two deaths from nodular and lentigo maligna melanoma) and seven with melanoma in situ (MIS). Invasive melanoma includes eight lentigo maligna melanoma, four nodular, two amelanotic and one desmoplastic. Mean Breslow thickness was 6 mm for invasive (range 0.5–26). Mean excision margin for MIS was 3 mm (range 2–5 mm) and for invasive was 5 mm (range 2–10). Further excisions were performed in nine (41%); two went on to recur. Local recurrence was 36%; six invasive (27%) at a mean of 24 months (range 1.5–5 years) and two for MIS at a mean of 15 months (range 1–1.5 years). Imaging occurred for suspected advanced disease. Sentinel node biopsy was not performed. Advanced melanoma therapy was performed in two cases. No vitamin D testing occurred.
Conclusions
Survival rates are in line with 90% overall survival in the UK. Prescriptive excision margins are not applicable in the periocular region and margin-controlled excision with a delayed repair is recommended, but patients need to know further excision may be needed to obtain clearance. Evidence recommending vitamin D therapy needs to be put into clinical practice. In addition, upstaging of MIS occurred advocating excision rather than observation of MIS. More studies are needed to determine the best management of eyelid melanoma.
Similar content being viewed by others
Introduction
Melanoma is one of the deadliest forms of skin cancer and is the fifth most common cancer worldwide. Melanoma of the head and neck has the highest mortality with eyelid representing the worst prognosis with a 10-year mortality of 90% [1]. The aetiology of melanoma is not fully understood, but risk factors comprise UV light exposure, previous sunburn (especially in childhood), history of non-melanoma skin cancer, advancing age, family history and genetic predisposition including Fitzpatrick skin type plus cutaneous cancer syndromes such as xeroderma pigmentosum [2]. Invasive melanoma more commonly arises de novo; however, eyelid melanoma is thought to arise from lentigo maligna, a form of melanoma in situ (MIS) [3].
Clinical diagnosis involves the ABCDE checklist of pigmented lesions (asymmetry, irregular border, multi-coloured, diameter >6 mm, elevation or evolving over time) with any suspicious lesions warranting biopsy. Histological diagnosis requires a full-thickness skin biopsy as the Breslow score is the most influential determinant of prognosis. Different histological subtypes harbour different BRAF and NRAS statuses, although their frequency in eyelid melanoma is unknown; invasive lentigo maligna melanoma is thought to be the most common subtype [4, 5]. Imaging is performed in the presence of nodal disease as a part of staging (stage III or IV) the patient. Surgery is the mainstay of treatment with UK excision margin recommendations of 0.5 cm for MIS, 1 cm for lesions <1 mm depth, 1–2 cm for lesion 1–2 mm thick, and 2–3 cm for thicker lesions [6]. Nevertheless, the trials that set these recommendations often excluded head and neck melanoma including eyelid melanoma [7]. Thus, excision margins are not feasible within the periocular region, increasing the risk of vision loss; hence, alternative margins are often used albeit not based on strong evidence. The eyelid melanoma working group suggest margins of <5 mm as probably being adequate for the periocular region, although those thicker than 1 mm (T2 or greater) probably warrants a wider excision [8]. These excision margins are based on consensus only and there is a dearth of evidence as to what excision margins should be used.
Advanced melanoma therapies have improved since immunotherapy became the mainstay of treatment. The advent of BRAF inhibitors improved life expectancy, paving the way for newer options such as checkpoint inhibitors, MEK inhibitors and the latest immunotherapy encompassing vaccine therapy, oncolytic viruses and T-cell engineering [9,10,11]. The use of these agents in eyelid melanoma is unknown. Cutaneous melanoma five-year survival for <1 mm thick lesions is over 90% whereas >4 mm thick conferring a 50% rate [12]. A small case series of 29 eyelid melanoma revealed 5 recurrences, 4 metastasis and 2 deaths; overall mortality is thought to be higher in eyelid melanoma compared to those elsewhere in the body [1, 5].
The objective of this study is to assess the tumour characteristics of eyelid melanoma over 5 years or more and compare the management to cutaneous melanoma standards, some of which may not be feasibly applicable to the periocular region. In particular, we wanted to assess how many of our cases would have been incompletely excised if we applied suggested excision margins for the eyelid region.
Patients and methods
A single-centre retrospective consecutive case review of 22 patients with eyelid melanomas. Institutional Review Board Committee approval (Queen Victoria NHS Trust Institutional Board number 1850) was obtained. Patients had their chart, histology and photographs reviewed. Consent was obtained for patient photographs. Patient demographics including ethnicity, age, gender, laterality, risk factors, histological subtype were recorded. Characterisation of the clinical presentation of eyelid melanoma was performed. A review was made to see if vitamin D levels were taken and any advice on supplementation given [13]. The technique used to excise both MIS and invasive melanoma was rush-paraffin, en face section margin-controlled excision with delayed repair (‘Slow Mohs’) and has been described elsewhere [14,15,16]. Briefly, mapped serial excision was employed with a narrow, pre-determined margin in order to preserve normal eyelid whilst ensuring safe clearance of the tumour. This form of margin-controlled excision used en face examination of the margin and subsequent delayed repair. The excision margin used in the removal of eyelid melanoma was pre-determined according to the staging of the melanoma. Further serial excisions were performed if there was a positive margin and continued until clearance of the tumour was obtained. The need for further excision required for tumour clearance was recorded. Reconstruction of the eyelid only occurred once the eyelid was histologically clear of tumour. Seventh edition cancer staging was used as all eyelid melanoma presented prior to the update [17]. We also recorded: nodal imaging, SLNB, BRAF status, use of genetic testing and novel therapies based on staging, visual outcome, disease progression, recurrence and survival.
Results
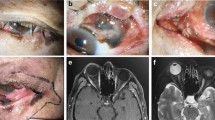
Over 10 years, 22 patients were identified including 7 females and 15 males (The patient data that support the findings of this study are available in Supplementary Table 1). The majority were on the lower eyelid (21) and only 2 on the upper. Representative images of the tumour at presentation are shown in Fig. 1. Histological diagnosis revealed 7 melanoma in situ (MIS), and 15 invasive melanoma with the majority of invasive being of the lentigo maligna melanoma subtype (8; see Fig. 2a). Two patients were diagnosed with MIS on biopsy, but were subsequently upstaged to invasive melanoma after formal excision. Mean excision margins used for MIS 3 mm (range 2–5 mm) and invasive disease 5 mm (range 2–10) (Fig. 2b). A 10 mm excision margin was used for a recurrent nodular melanoma on the eyelid cheek junction for an elderly gentleman to reduce multiple excisions. Further excisions were performed in 9 (41%); 2 went on to recur: if prescriptive eyelid melanoma excision margins were used (and no further excisions performed), then incomplete clearance would have occurred in 4 MIS and 5 invasive melanoma. The mode number of excisions or stages required for clearance was 2; however, this is only in a small sample size of 9 further excisions.
a Lentiga maligna melanoma in situ (MIS) of the left lower eyelid. b Right upper eyelid melanocytic naevus and right lower eyelid lentigo maligna invasive melanoma. c Right upper eyelid amelanotic nodular invasive melanoma (T4bN3M1b Breslow 6 mm BRAF/NRAS negative). d Nodular invasive melanoma (pT4bN2bM1d-Breslow 16 mm Wildtype BRAF/mutant NRAS. NRAS mutant exon3 codon 61: potential for MEK inhibitor).
Both Breslow and mitotic scores were not unanimously prognostic for recurrence, or mortality (Fig. 3a, b). Mean Clark level was 4 (range 3–5). There were two ulcerations, two perineural/lymphovascular invasions, four brisk infiltrating lymphocytes and four regression, although not significantly helpful for prognosis either. Seven patients underwent genetic testing with two tumours containing actionable genes (one BRAF and one NRAS); however, the mutant BRAF (c.1790T>A;p(Leu597Gin), was not located at V600, therefore was not eligible for a BRAF inhibitor. Imaging occurred for node/advanced disease. SLNB was not performed. Advanced melanoma therapy occurred in one case whereby a nodular melanoma received a checkpoint inhibitor Nivolumab.
Eight recurrences occurred in 6 patients with invasive melanoma (27% recurrence rate). Three were lentigo malignant melanoma (1 recurred at 3, 4 and 5 years; 1 had recurrent MIS-LM 3 years later; 1 had MIS-LM 2 years). Two were nodular melanoma (1 recurred at 1.5 years; 1 recurred at 2 years). One was a desmoplastic melanoma with recurrence of MIS-LM at 3 years. Of those that recurred there was one death. Two MIS recurred at 1 and 1.5 years (29% local recurrence for MIS). All first recurrences occurred within 5 years. Overall recurrence was therefore 8 (36%). Overall survival rate of 91% with a minimum of 5-year follow-up (range 5–12 years; 2 deaths: one nodular and one LMM directly related to metastatic melanoma). No vitamin D blood level testing occurred, and no advice was documented.
Discussion
In our case series, we found there to be a male preponderance which fits with published incidence data demonstrating a higher lifetime incidence in men for both invasive melanoma and MIS such that resources should be focused on identifying disease in the older male [18,19,20,21]. The incidence of melanoma has increased with MIS increasing the most; however, mortality has started to decrease in the last decade [19]. Survival rates in our cohort are in line with 90% cutaneous melanoma survival rates in the UK, however, this includes seven cases of MIS; 87% survival if you just look at invasive melanoma alone [22, 23]. Lower 5-year overall survival (OS) is noted for the eyelid region: a recent US study based on STEER data demonstrated 77% OS for invasive disease and 89% for MIS, but this includes antiquated cases from 1975 when survival rates were poorer [24]. Eyelid melanoma OS ranges from 77 to 88% for invasive disease and 74 to 89% for MIS [25, 26]. A minimum of 5 years of data are necessary as 90% of metastasis occurs within this time frame [27]. The local recurrence rate is higher than those quoted for cutaneous melanoma which ranges from as low as 2.9%, 9.4% if localising to the head and neck, and up to 40%, but is clearly dependent on staging [28,29,30,31]. Data on eyelid melanoma recurrence is sparse and are not quoted in either epidemiological studies or the STEER database. In 29 case reviews in Australia, a local recurrence rate of 17% was revealed, but with a median follow-up of 3 years [5]. In contrast, in 44 cases from the collaborative eyelid skin melanoma group, 25% had a local recurrence—a similar figure to our group [5, 8]. Time to first recurrence was all within the first 5 years. Conveying this to the patient should highlight the benefit of 5 years of surveillance.
Lentigo maligna has long been associated with the periocular region and the majority of invasive disease was of the lentigo malignant melanoma (LMM) subtype. A case series of 29 cases in Australia revealed a predominance of LMM (65%; 19 cases) and in ours was 53% of invasive tumours [5]. This contrasts with other parts of the body where superficial spreading (41%) and nodular (16%) predominate with LMM 14% or less [27]. Although it is said that invasive melanoma rarely arises de novo, it is stated that invasive head and neck LMM is thought to arise from in situ disease; we could not discern whether our LMM cases arose de novo or from MIS. The possible behaviour of LMM may explain the higher local recurrence rates compared to other parts of the body where it is a less common histological subtype.
Upstaging did occur in two cases whereby the biopsy revealed Mis but histological examination of the whole excised lesion revealed invasive disease; both were in the LMM subtype. Nevertheless, the natural history of Mis in sun-exposed regions is not clear and that LM Mis tends to have poorly defined edges occurring in sun-damaged skin plus subclinical spread [32]. Incidence data reveals that MIS OS rate is not 100% over 5 years advocating excision rather than observation. Nevertheless, it is probably better to carry out a more personalised approach taking other factors rather than a blanket advice to excise. Mistreatment is still controversial within the eyelid region as excision is a destructive process especially in larger lesions. The advice for Mis ranges from a watch and wait strategy to excision with a 5–10 mm margin strategy, imiquimod or radiotherapy [32,33,34].
Mean initial excision margin for the invasive disease was in line with the collaborative eyelid melanoma working group guidelines from 2003 whereby thin melanoma was recommended a <5 mm excision margin, but those with a Breslow thickness greater than 2 mm requiring >5 mm margin. However, if prescriptive excision margins of 3 and 5 mm were used for MIS and invasive disease, respectively, and without the opportunity to excise further, then incomplete clearance would have occurred in 41% including five invasive cases. Current international excision margin guidelines for cutaneous melanoma have reduced from a 2–4 cm excision margin to recommending no more than 2 cm even in thick tumours despite one RCT trial demonstrating that 1 cm was inadequate for Breslow greater than 2 mm [35, 36]. However, 2 cm is massive for the periocular region and not practically applicable.
The need for further excisions to minimise the amount of normal tissue that is removed needs to be conveyed to patients undergoing treatment. This is higher than cutaneous melanoma elsewhere, probably since smaller excision margins are applied to the eyelid in an attempt to preserve the greatest amount of normal eyelid as possible. Moreover, two of the immediate further excision patients went on to locally recur.
Breslow thickness has been identified as the most prognostic factor to immediate staging of the patient and for long-term prognosis. However, in our small study, the correlation between Breslow thickness was not as strong and two tumours less than 4 mm resulted in mortality. Furthermore, the majority of our patients had a Breslow depth above 2 mm which seems thicker than other studies report. Mitotic index may be slightly more correlative, but three had a high initial mitotic index over 10 per mm2 but did not result in mortality; however, it must be stressed that this is in a small number of patients. It could be that certain subtypes that are good at local invasion may not also be good at seeding elsewhere: their genetic profile is likely to be a better-determining factor. It has been suggested that Breslow density as well as depth can be used as a cheap biomarker of prognosis of mortality risk although was just outside of significance for overall survival on a multivariant analysis [37]. Nevertheless with newer, albeit more expensive whole genome sequencing of the tumour, we may be able to predict better those tumours that will metastasise or be more locally aggressive [38]. Genetic testing of the tumour in our group involved the identification of BRAF or NRAS allowing the patient to access BRAF inhibitors (vemurafenib, dabrafenib, and encorafenib) or MEK inhibitors (trametinib, binimetinib, selumetinib, and cobimetinib), respectively. The use of checkpoint inhibitor immunotherapy is independent of the tumour’s genetic profile (at this stage) and one patient received nivolumab for their third recurrence at 5 years since their primary and is currently in remission one year after administration.
Sentinel lymph node biopsy (SLNB) was not performed in our patients mainly as the patients were staged prior to the advent of novel therapies which are now available for node-positive disease and as such SLNB was mainly considered an academic or prognostic tool. Presently, however, the identification of micro-metastasis disease shifts the patient into stage 3 and allows them to gain access to adjuvant therapy; thus, it is recommended for those who are T2a or T1b with lymphovascular invasion and/or mitotic rate ≥2 mm2 [39]. Since the study concluded in 2016, SLNB has been routinely offered and performed for patients with a melanoma 0.8 mm or thicker on initial biopsy.
Vitamin D evidence needs to be integrated into daily practice and this was not the case as recommended in the NICE guidelines for melanoma [40]. Vitamin D has been shown to be beneficial in vitro, as prevention and we await the outcome of ViDe trial to better determine its effect on recurrence and survival [41,42,43,44].
Summary
What was known before
-
Melanoma is one of the deadliest forms of skin cancer.
-
Head and neck melanoma is said to have the highest mortality.
-
Few studies are in the literature on eyelid melanoma survival and prognosis.
-
Recommended excision margins are 1–2 cm for cutaneous melanoma.
-
Eyelid melanoma excision margins are based on consensus only and suggest 3 and 5 mm margins for MIS and invasive melanoma, respectively.
What the study adds
-
Overall survival for eyelid melanoma is in line with cutaneous melanoma elsewhere on the body at 90%.
-
Breslow depth and mitotic index may not be as prognostic within the eyelid region.
-
Excision margins were not between 1 and 2 cm as recommended for cutaneous melanoma; however, the overall mortality was not higher.
-
Prescriptive excision margins of 3 and 5 mm for MIS and invasive melanoma are not applicable in the periocular region otherwise there would have been incomplete clearance in 41% of cases.
-
Margin-controlled excision using slow Mohs technique is recommended with a clear explanation to the patient that further excision is commonly required to obtain tumour clearance.
-
Eyelid local recurrence rate is high at 36% and this may reflect the smaller excision margins used or the nature of the predominate histological subtype (Lentigo maligna MIS/melanoma) that may be more locally invasive.
-
First recurrence for both invasive and MIS all occurred within 5 years of the first excision.
-
Vitamin D levels need to be put into practice as the evidence shows that it is likely to reduce recurrence and improve overall survival.
Data availability
The patient data that support the findings of this study are available in Supplementary Table 1.
References
Larson DL, Larson JD. Head and neck melanoma. Clin Plast Surg. 2010;37:73–77.
Sanchez R, Ivan D, Esmaeli B. Eyelid and periorbital cutaneous malignant melanoma. Int Ophthalmol Clin. 2009;49:25–43.
Shields CL, Kels JG, Shields JA. Melanoma of the eye: revealing hidden secrets, one at a time. Clin Dermatol. 2015;33:183–96.
Saldanha G, Potter L, Daforno P, Pringle JH. Cutaneous melanoma subtypes show different BRAF and NRAS mutation frequencies. Clin Cancer Res. 2006;12:4499–505.
Chan FM, O’Donnell BA, Whitehead K, Ryman W, Sullivan TJ. Treatment and outcomes of malignant melanoma of the eyelid: a review of 29 cases in Australia. Ophthalmology. 2007;114:187–92.
Ahmed OA, Kelly C. Head and neck melanoma (excluding ocular melanoma): United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S133–S141.
Veronesi U, Cascinelli N, Adamus J, Balch C, Bandiera D, Barchuk A, et al. Thin stage I primary cutaneous malignant melanoma. Comparison of excision with margins of 1 or 3 cm. N Engl J Med. 1988;318:1159–62.
Esmaeli B, Youssef A, Naderi A, Ahmadi MA, Meyer DR, McNab A. Margins of excision for cutaneous melanoma of the eyelid skin: the Collaborative Eyelid Skin Melanoma Group Report. Ophthalmic Plast Reconstr Surg. 2003;19:96–101.
Zhu Z, Liu W, Gotlieb V. The rapidly evolving therapies for advanced melanoma–towards immunotherapy, molecular targeted therapy, and beyond. Crit Rev Oncol Hematol. 2016;99:91–99.
Kim A, Cohen MS. The discovery of vemurafenib for the treatment of BRAF-mutated metastatic melanoma. Expert Opin Drug Discov. 2016;11:907–16.
Michielin O, Hoeller C. Gaining momentum: new options and opportunities for the treatment of advanced melanoma. Cancer Treat Rev. 2015;41:660–70.
Saranga-Perry V, Ambe C, Zager JS, Kudchadkar RR. Recent developments in the medical and surgical treatment of melanoma. CA Cancer J Clin. 2014;64:171–85.
National Institute for Health and Care Excellence. Melanoma: assessment and management. https://www.nice.org.uk/guidance/ng14.
Malhotra R, Chen C, Huilgol SC, Hill DC, Selva D. Mapped serial excision for periocular lentigo maligna and lentigo maligna melanoma. Ophthalmology. 2003;110:2011–8.
Huilgol SC, Selva D, Chen C, Hill DC, James CL, Gramp A, et al. Surgical margins for lentigo maligna and lentigo maligna melanoma: the technique of mapped serial excision. Arch Dermatol. 2004;140:1087–92.
Then S-Y, Malhotra R, Barlow R, Kurwa H, Huilgol S, Joshi N, et al. Early cure rates with narrow-margin slow-Mohs surgery for periocular malignant melanoma. Dermatol Surg. 2009;35:17–23.
Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472–92.
Higgins HW II, Lee KC, Galan A, Leffell DJ. Melanoma in situ: Part I. Epidemiology, screening, and clinical features. J Am Acad Dermatol. 2015;73:181–90.
Kurtansky NR, Dusza SW, Halpern AC, Hartman RI, Geller AC, Marghoob AA, et al. An epidemiologic analysis of melanoma overdiagnosis in the United States, 1975-2017. J invest Dermatol. 2021;142:1804–11.e6.
Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29.
Chen ST, Geller AC, Tsao H. Update on the epidemiology of melanoma. Curr Dermatol Rep. 2013;2:24–34.
CRUK. Melanoma skin cancer mortality. 2021.
Downing A, Newton-Bishop JA, Forman D. Recent trends in cutaneous malignant melanoma in the Yorkshire region of England; incidence, mortality and survival in relation to stage of disease, 1993-2003. Br J Cancer. 2006;95:91–5.
Go CC, Kim DH, Go BC, McGeehan B, Briceño CA. Clinicopathologic characteristics and prognostic factors impacting survival in melanoma of the eyelid. Am J Ophthalmol. 2022;234:71–80.
Yin VT, Warneke CL, Merritt HA, Esmaeli B. Number of excisions required to obtain clear surgical margins and prognostic value of AJCC T category for patients with eyelid melanoma. Br J Ophthalmol. 2014;98:1681–5.
Jung S-K, Lim J, Yang S-W, Jee D, Won Y-J. Nationwide trends in the incidence and survival of eyelid skin cancers in Korea. Ophthalmic Epidemiol. 2020;27:438–48.
Garbe C, Amaral T, Peris K, Hauschild A, Arenberger P, Bastholt L, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: diagnostics - Update 2019. Eur J Cancer. 2020;126:141–58.
Karakousis CP, Balch CM, Urist MM, Ross MM, Smith TJ, Bartolucci AA. Local recurrence in malignant melanoma: long-term results of the multiinstitutional randomized surgical trial. Ann Surg Oncol. 1996;3:446–52.
Balch CM, Soong SJ, Smith T, Ross MI, Urist MM, Karakousis CP, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1-4 mm melanomas. Ann Surg Oncol. 2001;8:101–8.
Gregory McKinnon J, Starritt EC, Scolyer RA, McCarthy WH, Thompson JF. Histopathologic excision margin affects local recurrence rate: analysis of 2681 patients with melanomas. Ann Surg. 2005;241:326–33.
Romano E, Scordo M, Dusza SW, Coit DG, Chapman PB. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J Clin Oncol. 2010;28:3042–7.
Friedman EB, Scolyer RA, Williams GJ, Thompson JF. Melanoma in situ: a critical review and re-evaluation of current excision margin recommendations. Adv Ther. 2021;38:3506–30.
Tzellos T, Kyrgidis A, Mocellin S, Chan A-W, Pilati P, Apalla Z. Interventions for melanoma in situ, including lentigo maligna. Cochrane Database Syst Rev. 2014:CD010308.
Hendrickx A, Cozzio A, Plasswilm L, Panje CM. Radiotherapy for lentigo maligna and lentigo maligna melanoma – a systematic review. Radiat Oncol. 2020;15:174.
Garbe C, Amaral T, Peris K, Hauschild A, Arenberger P, Bastholt L, et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: treatment - update 2019. Eur J Cancer. 2020;126:159–77.
Hayes AJ, Maynard L, Coombes G, Newton-Bishop J, Timmons M, Cook M, et al. Wide versus narrow excision margins for high-risk, primary cutaneous melanomas: long-term follow-up of survival in a randomised trial. Lancet Oncol. 2016;17:184–92.
Secinti IE, Gursoy D, Erturk T, Dede I, Ozgur T, Dogan E. Should we report Breslow density, a new concept in cutaneous melanoma? Malays J Pathol. 2021;43:397–404.
Guhan S, Klebanov N, Tsao H. Melanoma genomics: a state-of-the-art review of practical clinical applications. Br J Dermatol. 2021;185:272–81.
Peach H, Board R, Cook M, Corrie P, Ellis S, Geh J, et al. Current role of sentinel lymph node biopsy in the management of cutaneous melanoma: a UK consensus statement. J Plast Reconstr Aesthet Surg. 2020;73:36–42.
Martin-Gorgojo A, Gilaberte Y, Nagore E. Vitamin D and skin cancer: an epidemiological, patient-centered update and review. Nutrients. 2021;13:4292.
De Smedt J, Van Kelst S, Boecxstaens V, Stas M, Bogaerts K, Vanderschueren D, et al. Vitamin D supplementation in cutaneous malignant melanoma outcome (ViDMe): a randomized controlled trial. BMC Cancer. 2017;17:562.
Cunha N, Campos S, Serrao V. Vitamin D levels in a cohort of Portuguese melanoma patients relate to time of follow-up from diagnosis, sun-exposure behaviour, and use of photoprotection. Eur J Dermatol. 2018;28:93–94.
Skobowiat C, Oak ASW, Kim T-K, Yang CH, Pfeffer LM, Tuckey RC, et al. Noncalcemic 20-hydroxyvitamin D3 inhibits human melanoma growth in in vitro and in vivo models. Oncotarget. 2017;8:9823–34.
Tang JY, Fu T, Leblanc E, Manson JE, Feldman D, Linos E, et al. Calcium plus vitamin D supplementation and the risk of nonmelanoma and melanoma skin cancer: post hoc analyses of the women’s health initiative randomized controlled trial. J Clin Oncol. 2011;29:3078–84.
Author information
Authors and Affiliations
Contributions
Design of study: JCB, AL, RM. Conduct of study, collection, management, analysis and interpretation of data: JCB, AL, RM. Manuscript preparation and final approval: JCB, AL, RM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bladen, J.C., Malhotra, R. & Litwin, A. Long-term outcomes of margin-controlled excision for eyelid melanoma. Eye 37, 1009–1013 (2023). https://doi.org/10.1038/s41433-023-02428-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02428-9
This article is cited by
-
Surgical margins and outcomes for eyelid melanoma: a systematic review and meta-analysis
Archives of Dermatological Research (2024)