Abstract
Background/Objectives
To report the incidence, microbiological profile and in-vitro antimicrobial susceptibilities of microbial keratitis (MK) in the East of England (EoE) over a 6-year period.
Subjects/Methods
A retrospective study of patients diagnosed with MK who underwent corneal scraping at participating trusts, within the EoE, between 01/01/2015–01/07/2020. Analysis was performed on MK isolate profiles, in-vitro anti-microbial sensitivities and trends over time.
Results
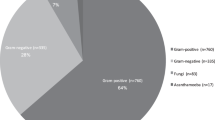
The mean incidence of IK, in the EoE, was estimated at 6.96 per 100 000 population/year. 1071 corneal scrapes were analysed, 460 were culture positive (42.95%) of which 87.2% were bacteria (50.3% gram-positive and 49.7% gram-negative), 2.4% polymicrobial, 9.3% fungi and 1.1% acanthamoeba. The most common organisms were pseudomonas spp (29.57%). There was a non-statistically significant trend (NST) in increasing incidence of pseudomonas spp, staph aureus and serratia (p = 0.719, p = 0.615, and p = 0.099 respectively) and a declining NST in Fungi (p = 0.058). Susceptibilities in-vitro to, penicillin classes, fluoroquinolone and aminoglycosides were 76.7% and 89.4%, 79.2% and 97.2% and 95.4 and 96.1% to gram-positive and gram-negative bacteria respectively. Gram-negative organisms were increasingly resistant to cephalosporins with a 19.2% reduction in sensitivity over time. (p = 0.011). Ceftriaxone showed the greatest decrease in sensitivity of 41.67% (p = 0.006).
Conclusion
In the EoE, MK is relatively prevalent though likely underestimated. Profiles are similar to other UK regions with the exception of a higher fungal and lower acanthamoeba incidence. Common first and second-line antimicrobial selection provides, on the whole, good coverage. Nevertheless, anti-microbial resistance, to cephalosporins, was observed so selection should be carefully considered when treating MK empirically.
Similar content being viewed by others
Introduction
Microbial keratitis (MK) remains a significant cause of ocular morbidity in the UK. With an incidence of 3.3–52.1 per 100,000, the condition is regularly seen in ophthalmic emergency services making up 3.3% of attendees [1,2,3,4].
Early treatment reduces the risk of corneal scarring, vascularisation, or perforation necessitating corneal transplant [5]. Treatment is initiated with broad spectrum empirical antibiotics, based on local epidemiological data, prior to targeted treatment guided by corneal scraping for gram-stain, culture and sensitivity [1, 5].
Studies in the UK have reported rising anti-microbial resistance, with a propensity toward harder to treat organisms varying by region [3, 6,7,8]. Accordingly, epidemiological studies to guide empirical anti-microbial choice are essential. MK isolates, their incidence and corresponding sensitivities have not been previously reported in the East of England (EoE).
We aim to report the incidence, microbiological profile and in-vitro antimicrobial susceptibilities of MK in the EoE for the past 6 years.
Materials/subjects and methods
A retrospective study of patients diagnosed clinically with infectious keratitis (IK) who subsequently underwent corneal scraping. Clinically suspected or PCR samples of viral keratitis were excluded. All corneal scrape specimens reviewed by microbiology departments in participating trusts within the EoE between 01/01/2015-01/07/2020 were included. Hospitals participating were: Broomfield, Cambridge-University Hospitals, Colchester, Hinchingbrooke, Ipswich, Southend-University Hospital, and Norfolk and Norwich University Hospitals.
A corneal scrape kit consists of a glass slide and several agar plates including: Blood, Chocolate (6% CO2, at 37 °C) and Sabouraud’s Dextrose Agar (air at 30 °C). Depending on clinical suspicion, in cases of contact lens (CL) IK, samples were sent for acanthamoeba PCR.
Corneal infective tissue was obtained with a sterile needle with separate instruments utilised for each culture medium to prevent cross-contamination whilst inoculating agar plates. Scrapes were taken prior to antibiotic installation unless treatment had been initiated by an external provider, or topical antibiotics withheld for 24 h if the scrape had to be repeated in cases of non-responsive keratitis. When repeat scrapes were taken, they were counted as a single episode and only included if the species or sensitivity differed from the first sample.
Data was collected using pro-forma including hospital trust, number, date of scrape, gram-stain, isolates and antimicrobial susceptibilities. Organisms were classified as gram-positive bacteria, gram-negative bacteria, acanthamoeba or fungi. We defined polymicrobial keratitis as MK caused by 2≤ organisms from a single episode of infection.
We determined the incidence of MK in the EoE by estimating the population size from 2015–2019. This was achieved, utilising Office of National Statistics Data [9]. We did not calculate an incidence for 2020, due to a lack of a full year worth of data.
As a retrospective chart review, ethics approval was not required. The study was conducted in accordance with the tenets of the Declaration of Helsinki
Statistical analysis
Analysis was performed using SPSS V.24.0 (IBM SPSS Statistics for Macintosh).
For the purposes of description and analysis of trends, the study period was divided into two groups of three years: 2015–2017 and 2018–2020.
As the data analysed was categorical, normality was not required to be assessed. Comparison between groups were reviewed using Pearson’s Chi- Square or Fisher’s exact test. When a variable was <5, Fisher’s was performed for comparison between groups. P value of ≤0.05 was considered statistically significant.
Results
Incidence and isolate trends
The mean incidence of IK, in the EoE, over the six-year period, was estimated at 6.96 per 100,000 population/year (95% CI, 3.35–10.56 per 100,000 population/year r = −0.029 p = 0.957). The incidence of IK was found to increase and then stabilise between 2015–2018 then decline in 2019 (Fig. 1).
1071 corneal scrapes were analysed with a mean patient age of 63.5 ± 19.1 (range 12.0–95.0) of which 53.2% were male and 46.8% female. Of 1071 scrapes, 460 were culture positive (42.95%) of which 87.2% were bacteria (50.3% gram-positive and 49.7% gram-negative), 2.4% polymicrobial, 9.3% fungi and 1.1% acanthamoeba (Fig. 2). From scrapes with sensitivity data, the average number of antimicrobials tested was 4.32 (range 1–12). We estimate 65.9% of our IK cases originating from rural localities calculated utilising Urban-Rural Classification Tables [10]. Each locality, served by participating hospitals, was categorised into either urban or rural. The percentage of rural regions as proportion of total localities generated an approximate figure for IK cases originating from rural localities.
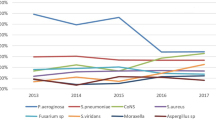
The most commonly isolated organisms were pseudomonas spp (29.57%), staphylococcus aureus (SA) (13.04%), coagulase negative staphylococcus (CNS) spp (8.70%) and streptococcus spp (7.39%). There was a non-statistically significant trend (NST) in increasing incidence of pseudomonas spp, SA, streptococcus spp and serratia over the study period (p = 0.719, p = 0.615, p = 0.575 and p = 0.099 respectively). Conversely, there was a declining NST in CNS and Fungi with the latter almost halving (p = 0.135 and p = 0.058 respectively). There was a NST doubling in the positive culture rates for acanthomoeba between the two time periods from 0.78% to 1.5% (p = 0.658) (Fig. 3).
Antimicrobial susceptibility
Gram-positive
Susceptibilities in-vitro to chloramphenicol, penicillin classes, fluoroquinolone, aminoglycosides and glycopeptides were 83.6% (92/110), 76.7% (56/73), 79.2% (42/53), 95.4% (104/109) and 93.5% (43/46) respectively (Figs. 4, 5). Average sensitivity of gram-positive organisms to common antimicrobial agents, which include glycopeptides, chloramphenicol and fluoroquinolones, is 84.9%. However, streptococcus spp were found to be less susceptible to aminoglycosides (66.7%) compared to other gram-positive organisms (≥90% p > 0.05). Organisms, such as SA and streptococcus spp, achieved 100% sensitivities to fluoroquinolones, whilst CNS and staphylococcus epidermidis were less susceptible (57.1% and 66.7% respectively p > 0.05).
Gram-negative
Susceptibilities in-vitro to cephalosporins, penicillin classes, fluoroquinolone, aminoglycosides and colistin were 91.8% (56/61), 89.4% (126/141), 97.2% (69/71), 96.1% (147/153) and 98.0% (49/50) respectively (Figs. 4, 5). The average sensitivity of gram-negative organisms to common antimicrobial agents, which include aminoglycosides, fluoroquinolones and cephalosporins, is 94.3%. However, serratia spp were less susceptible to cephalosporins (66.6%) compared to other gram-negative organisms (≥90% p > 0.05).
Fungi
All fungi, were found to be susceptible to all anti-fungal agents tested (Figs. 4, 5).
Trends
Antimicrobial sensitivity to gram-positive and gram-negative organisms remained stable as a NST (p = 0.990 and p = 0.418). Gentamicin, fluoroquinolones and clindamycin were possibly less effective against gram-positive organisms over time with a 3.8%, 4.1% and 20.4% reduction in effectiveness respectively (p = 0.621, p = 0.732 and p = 0.182). Gram-negative organisms were increasingly resistant to cephalosporins, a 19.2% reduction in sensitivity over the study period. (p = 0.011).
Cephalosporins under investigation were: ceftriaxone, ceftazadime, cefpodoxime and cefuroxime. Ceftriaxone showed the greatest decrease in sensitivity of 41.67% (p = 0.006). Other cephalosporins investigated maintained sensitivities of 100%, to gram-negative organisms over time.
Penicillin class antimicrobials, aminoglycosides and fluoroquinolones appeared to remain effective against gram-negative organisms (p = 0.078, p = 0.214 and p = 1.000 respectively) (Fig. 4).
Discussion
MK remains a challenge to countries across the globe [11]. This is the first study reporting the incidence, isolate profiles and their in-vitro anti-microbial susceptibilities in EoE.
We found, 42.95% of corneal scrapes were culture positive, in range of other published studies (32.6–54%) [3, 6, 7, 11, 12]. Low culture-positivity may be attributed to a low threshold for performing scrapes which were encouraged at our institutions leading to samples being taken for non-infectious aetiologies e.g. marginal keratitis. Low yields may also be a consequence of: poor technique, inadequate sampling and antimicrobial use, such as chloramphenicol, in primary care and A&E, prior to scraping.
Incidence
A range of estimates exist for IK incidence across the UK. Urban and Rural localities, such as Nottingham and the West of Scotland have widely different values at 34.7 and 3.3 per 100,000/year respectively [2, 3, 13]. Our study found a rate comparable to the latter at 6.96 per 100,000 population/year [2, 3, 13]. However, this could be an underestimation for several reasons. Peripheral and small ulcers, may have been treated empirically without scraping. A degree of cross-cover exists from hospitals in other regions. Any data from EoE patients, treated elsewhere would be excluded. Finally, we found the incidence of IK declining in 2019 during the COVID-19 Pandemic. This is likely due to a well-documented, reduction in the presentation of ocular emergencies including MK [14].
Ting and colleagues noticed a stable trend in IK cases in Nottingham whilst Ibrahim et al found an upward trend in Portsmouth. Our review identified an increasing trend akin to the latter study till the pandemic [2, 3]. Hypotheses’ to explain this trend include: an increasing uptake of CL wear in the population [15], and an increasing number of elderly patients, with ocular comorbidities, who are predisposed to IK from serratia or Moraxella [16,17,18,19].
Variations of IK between urban and rural localities may be explained by CL wear, age, deprivation, demographics and co-morbidities. Older individuals are less likely to opt for CL wear with peak usage between the ages of 25–44. The EoE and other rural localities have older populations compared to other UK regions [20, 21]. A lack of CL use, relative to the population average, may help to explain the lower incidence of IK in rural localities.
Isolate profiles
Pathogens, responsible for MK, vary with climate, region and demographics [8]. Bacteria and Fungi are the commonest isolates in developed and developing nations respectively. Several factors including population behaviours, e.g., CL use, climate and occupational differences may explain this disparity [8].
In our study, the most common isolates were bacteria (89.6%) followed by fungi (9.3%) the former is comparable but the latter is higher than equivalent UK studies [3, 6, 7]. This may be attributed to our region having a large rural farming population where vegetative trauma associated injuries are more prevalent compared to urban localities [10].
Within bacterial isolates, we observed gram-positive bacteria, as most prevalent responsible for 50.4% of all bacterial isolates, in-line with other UK reviews. Nevertheless, the magnitude of difference, between gram-positive and negative organisms, was smaller relative to any other study [3, 6, 7, 11, 12]. Some studies, performed years earlier, had shown a decreasing trend of gram-positive organisms whilst gram-negatives remained stable or even increased [6, 12]. Our study perhaps shows the consequence of these long-term trends identified earlier.
Of gram-positive isolates, SA was most common followed by CNS in contrast to older studies where CNS was most prevalent [2, 7, 11, 12]. Recent studies have shown a declining trend of CNS with an increasing trend of SA with others finding SA to be the commonest gram-positive organism [3, 6]. The pathogenic role of CNS, in immunocompetent patients, has been controversial. Determining between pathogenic isolates and contaminants remains challenging. A declining trend in CNS could be due to improved acquisition and laboratory technique, reducing commensal contamination of samples [22].
Previous UK studies, identified gram-positive organisms, most commonly CNS, to be most prevalent [6, 7, 11, 12]. However, our results identified Psuedomonas spp to be most common (29.57%) as per other recent studies (23.6%) [3]. Earlier reviews have shown a declining trend of gram-positive organisms with an increasing number of gram-negative with pseudomonas as the commonest gram-negative species. Recent papers, such as ours, possibly show the culmination of these long-term trends as a consequence of greater CL wear in the population [3, 6, 12, 15, 19].
Authors have noted an increasing trend or stable infection rates of moraxella species in different UK regions [3, 6, 7, 11, 12]. Contrary to this, our data shows a decline in moraxella (p = 0.308) and an increase in serratia keratitis (5.2% p = 0.099). Both serratia and moraxella species afflict the elderly, those with ocular surface disease and on multiple topical treatments e.g., glaucoma patients [16, 17]. Like other regions in the UK, we have seen rising numbers of such patients due to an ageing population with complex health needs, explaining an increase in both serratia and moraxella cases. However, serratia tends to thrive in water and soil whilst moraxella is found in the Upper Respiratory Tract and flourishes in colder environments [18, 23] Several studies, noting a rise in Moraxella, cases were performed in large urban centres such as Nottingham and Manchester, favouring moraxella infection over serratia in their at-risk groups. In the EoE, with our large rural populations, the inverse may be true, explaining our findings.
We identified a 5.2% reduction in fungal isolates (p = 0.058) consistent with the literature, where a similar pattern of increasing yeast coupled with a decline in filamentous isolates resulted in a net reduction of fungal keratitis [11]. Other papers have shown stability of fungal keratitis or an increasing trend [3, 6, 12]. Risk factors for yeast keratitis (YK) include, poor ocular surface and chronic steroid use whilst for Fusarium spp, a common cause of filamentous keratitis (FK), trauma and CL use are risk factors [24, 25] The decline in FK observed may be due to a relative reduction in CL wear during the pandemic coupled with fewer vegetation associated traumatic injuries influenced by lockdowns. These changes, alongside the long-term trend of elderly patients, with chronic ocular co-morbidities, may explain the rise in YK.
We identified 1.1% of acanthamoeba cases in culture positive samples an underestimate compared to other studies [3, 6, 11]. This may be explained by the relatively low sensitivity of cultures compared to confocal microscopy and PCR [26, 27]. Like other studies, we noticed an increase in acanthamoeba keratitis (AK) between the study periods (p = 0.658) [6, 7, 11]. This is likely related in part to an absolute increase in CL wearers in all populations but also because trends in CL prescribing show an increase in the average age of CL wearers, who may have more susceptibility to microbial keratitis [15, 19, 28, 29]. The EoE, with its on average older populations, may be experiencing rapid adoption of CL wear in middle aged patients compared to more urban populations [20, 21].
Anti-microbial resistance
Rising antimicrobial resistance is designated as a significant risk by governments [30]. Ocular infections have not been exempt from this emerging threat [31].
Both groups of gram-positive and gram-negative organisms likely maintained their sensitivities to antimicrobials (p = 0.990 and 0.418 respectively) consistent with other studies [6, 12]. It is reassuring that common, over the counter, antimicrobials such as chloramphenicol and fusidic acid remain effective (p = 0.335 and p = 0.219 respectively) also seen in other studies [6, 7, 12]. Dispensing of such antimicrobials by GPs and A&E staff, in presumed IK whilst awaiting specialist input, should continue and be encouraged.
Corroborating other reviews, gram-positive bacteria achieved moderate susceptibility to fluroquinolones whilst gram-negative were highly susceptible. Glycopeptides and aminoglycosides were effective against both species [3, 6, 7, 31]. In EoE, first-line treatment entails monotherapy with a fluroquinolone and monitoring. This strategy appears reasonable considering the 79.2% and 97.2% effectiveness against gram-positive and gram-negative organisms respectively. Second-line treatments, involving an aminoglycoside and glycopeptide, in conjunction, achieve a > 93% susceptibility against all bacteria.
Although in-vitro testing is correlated to therapeutic response, it’s only an estimate to clinical response. In-vivo antibiotics applied topically and intensively may achieve higher concentrations than those seen in-vitro with greater bactericidal effect. Reported in-vitro susceptibility figures could be an underestimate of in-vivo real-world results [6, 32].
Alternative second-line treatment options include a cephalosporin and aminoglycoside [6]. Studies have shown cephalosporins displaying weaker efficacy to gram-negative organisms and increasing resistance, though without significance [3, 12, 30]. Our study identified a 19.2% increase in resistance to cephalosporins over time (p = 0.011) which originated from ceftriaxone with a 41.67% decline in sensitivity (p = 0.006). Other cephalosporins investigated retained a 100% sensitivity to gram-negative organisms. Emerging trends of gram-negative resistance to ceftriaxone, has been previously identified [4]. In one study, 79.2% of MDR pseudomonas displayed resistance to ceftriaxone; the highest combined rates of antibiotic resistance [33]. Cephalosporins, such as ceftazidime, and aminoglycoside may still be an effective second line choice, though careful selection of the generation and type of cephalosporin should be considered.
Microbiology sensitivity testing is often incongruous with clinical need. Of the 622 anti-microbial sensitivity tests on gram-positive organisms, only 55 were to fluroquinolones and a mere six on cephalosporins. In clinical practice, fluroquinolones and cephalosporins are used as first and second line agents respectively [1, 5]. Standardisation of sample collection and testing protocols between microbiology and ophthalmic departments would improve culture positivity and ensure sensitivity data has greater relevance to clinicians.
Limitations
The study’s conclusions must be interpreted in the context of its methodological limitations.
Whilst similar papers have collected data from a single institution, we have sourced anonymized records from several independent trusts. Although the principles of investigation and treatment are similar, regional variations in protocols, guidelines, information governance and testing exist. The variation in information governance protocols between trusts posed challenges when requesting demographic data, resulting in either incomplete or no demographic data being provided. As microorganisms vary according to locality, extrapolation of our findings to different settings must be done with care. Certain organisms identified, such as CNS and staph epidermidis, could represent commensals from the ocular surface and surrounding tissues. Without clinical correlation, contextualising their pathogenicity or commensal nature was challenging. Our study was not intended to include clinical outcomes of IK. Selective testing of isolates to antimicrobials based on prior knowledge of susceptibilities could introduce selection bias and skew results. In-vitro testing of susceptibilities may underestimate true efficacy in-vivo. Serum mean inhibitory concentrations, utilised in-vitro, may be unrepresentative, as higher concentrations of antimicrobial are delivered to the site of action clinically. Nevertheless, studies have shown reasonable correlation between in-vitro testing and clinical outcomes [34]. Finally, changes in societal behaviours and services under pressure during the pandemic, may have led to an underestimation of MK, organisms cultured and sensitivity profiles in the latter half of the study.
Conclusion
Firstly, we have provided an up-to-date estimate of IK incidence in the EoE at 6.96 per 100,000 population/year. Secondly, we found, gram-positive organisms to be the commonest cause of IK though pseudomonas was the commonest organism. Thirdly, a NST of rising serratia and acanthamoeba keratitis has been detected in the region with a fall in fungal keratitis. Finally, over the counter anti-microbials maintain their effectiveness against bacteria as do common first line agents such as fluroquinolones. Second line antimicrobials such as glycopeptide and aminoglycoside combinations also maintain their effectiveness and are reasonable choices. Nevertheless, gram-negative organisms exhibited increasing resistance to ceftriaxone. Cephalosporin selection should be carefully considered when treating IK empirically.
Summary
What was known before
-
Infectious Keratitis (IK) incidence, isolates, trends and resistance patterns do vary between regions although on the whole are comparable. No data on infectious keratitis isolates, trends and resistance patterns have been published in the East of England region.
What this study adds
-
Incidence of IK is estimated at 6.96 per 100,000 population/year in the region. Isolate profiles, trends and sensitivities, overall, are comparable with other regions of the UK. Although, a significant rising trend of ceftriaxone resistance to gram negative organisms were identified. IK remains a significant cause of morbidity in the region. Common 1st/2nd line agents remain effective against typical pathogens although cephalosporin selection must be carefully evaluated when treating IK empirically.
Data availability
Raw data can be made available on reasonable request.
References
Ung L, Bispo PJM, Shanbhag SS, Gilmore MS, Chodosh J. The persistent dilemma of microbial keratitis: global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2019;64:255–71. https://doi.org/10.1016/j.survophthal.2018.12.003.
Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93:1319–24.
Ting DSJ, Ho CS, Cairns J, Elsahn A, Al-Aqaba M, Boswell T, et al. 12-year analysis of incidence, microbiological profiles and in vitro antimicrobial susceptibility of infectious keratitis: the Nottingham Infectious Keratitis Study. Br J Ophthalmol. 2021;105:328–33. https://doi.org/10.1136/bjophthalmol-2020-316128
Paterson DL, Henderson A, Harris PNA. Current evidence for therapy of ceftriaxone-resistant Gram-negative bacteremia. Curr Opin Infect Dis. 2020;33:78–85.
Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124:1678–89.
Tan SZ, Walkden A, Au L, Fullwood C, Hamilton A, Qamruddin A, et al. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye (Lond). 2017;31:1229–36.
Tavassoli S, Nayar G, Darcy K, Grzeda M, Luck J, Williams OM, et al. An 11-year analysis of microbial keratitis in the South West of England using brain-heart infusion broth. Eye (Lond). 2019;33:1619–25. https://doi.org/10.1038/s41433-019-0463-6.
Shah A, Sachdev A, Coggon D, Hossain P. Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. Br J Ophthalmol. 2011;95:762–7. https://doi.org/10.1136/bjo.2009.169607.
Office for National Statistics Estimates of the population for the UK, England and Wales, Scotland and Northern Ireland - Office for National Statistics. (2022) [online] Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland.
Department for Environment, Food & Rural Affairs Rural Urban Classification lookup tables for all geographies (2011) [online] Available at: https://www.gov.uk/government/statistics/2011-rural-urban-classification-lookup-tables-for-all-geographies
Ting DSJ, Settle C, Morgan SJ, Baylis O, Ghosh S. A 10-year analysis of microbiological profiles of microbial keratitis: the North East England Study. (Lond). 2018;32:1416–7.
Orlans HO, Hornby SJ, Bowler IC. In vitro antibiotic susceptibility patterns of bacterial keratitis isolates in Oxford, UK: a 10-year review. Eye (Lond). 2011;25:489–93.
Seal DV, Kirkness CM, Bennett HG, Peterson M. Population-based cohort study of microbial keratitis in Scotland: incidence and features. Cont Lens Anterior Eye. 1999;22:49–57.
Wickham L, Hay G, Hamilton R, Wooding J, Tossounis H, da Cruz L, et al. The impact of COVID policies on acute ophthalmology services-experiences from Moorfields Eye Hospital NHS Foundation Trust. Eye (Lond). 2020;34:1189–92.
Euromcontact A comparison of European Soft contact Lens and Lens Care Markets in 2021 Euromcontact (2021). [online] https://www.statista.com/statistics/431231/penetration-of-individuals-who-wear-contact-lens-by-type-of-lens-in-the-uk-and-ireland/
Das S, Constantinou M, Daniell M, Taylor HR. Moraxella keratitis: predisposing factors and clinical review of 95 cases. Br J Ophthalmol. 2006;90:1236–8. https://doi.org/10.1136/bjo.2006.095182.
Mah-Sadorra JH, Najjar DM, Rapuano CJ, Laibson PR, Cohen EJ. Serratia corneal ulcers: a retrospective clinical study. Cornea. 2005;24:793–800.
Equi RA, Green WR. Endogenous Serratia marcescens endophthalmitis with dark hypopyon: case report and review. Surv Ophthalmol. 2001;46:259–68.
Association of Contact Lens Manufacturers ACLM Market Report 2014 Optical Confederation (UK) (2014) [online] Available at: https://www.statista.com/statistics/429790/wearers-of-contact-lenses-united-kingdom-ireland/.
BMG research Contact Lens Survey 2015 General Optical Council (2015) [online] Available at: https://www.statista.com/statistics/666025/contact-lens-wearers-by-age-united-kingdom-uk/
Office for National Statistics (UK) Population estimates for the UK. Office for National Statistics (UK) (2021) [online] Available at: https://www.statista.com/statistics/367796/uk-median-age-by-region/
Piette A, Verschraegen G. Role of coagulase-negative staphylococci in human disease. Vet Microbiol. 2009;134:45–54.
Walkden A, Fullwood C, Tan SZ, Au L, Armstrong M, Brahma AK, et al. Association between season, temperature and causative organism in microbial keratitis in the UK. Cornea. 2018;37:1555–60.
Hoffman JJ, Burton MJ, Leck A. Mycotic keratitis-a global threat from the filamentous fungi. J Fungi (Basel). 2021;7:273.
Acharya Y, Acharya B, Karki P. Fungal keratitis: study of increasing trend and common determinants. Nepal J Epidemiol. 2017;7:685–93. https://doi.org/10.3126/nje.v7i2.17975.
Goh JWY, Harrison R, Hau S, Alexander CL, Tole DM, Avadhanam VS. Comparison of in vivo confocal microscopy, PCR and culture of corneal scrapes in the diagnosis of acanthamoeba keratitis. Cornea Published. 2018;37:480–5.
Hoffman JJ, Dart JKG, De SK, Carnt N, Cleary G, Hau S. Comparison of culture, confocal microscopy and PCR in routine hospital use for microbial keratitis diagnosis. Eye (Lond). 2022;36:2172–8. https://doi.org/10.1038/s41433-021-01812-7.
Morgan PB, Efron N. Global contact lens prescribing 2000–2020. Clin Exp Optom. 2022;105:298–312. https://doi.org/10.1080/08164622.2022.2033604. Published
Radford CF, Minassian DC, Dart JK. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol. 2002;86:536–42. https://doi.org/10.1136/bjo.86.5.536.
Department of Health and Social Care UK 5-year action plan for antimicrobial resistance 2019 to 2024 HM Government Publications (2019) [online] Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/784894/UK_AMR_5_year_national_action_plan.pdf.
Ting DSJ, Ho CS, Deshmukh R, Said DG, Dua HS. Infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye (Lond). 2021;35:2908.
Wilhelmus KR, Abshire RL, Schlech BA. Influence of fluoroquinolone susceptibility on the therapeutic response of fluoroquinolone-treated bacterial keratitis. Arch Ophthalmol. 2003;121:1229–33.
Hadadi-Fishani M, Khaledi A, Fatemi-Nasab ZS. Correlation between biofilm formation and antibiotic resistance in Pseudomonas aeruginosa: a meta-analysis. Infez Med. 2020;28:47–54.
Chen A, Prajna L, Srinivasan M, Acharya N, Lietman T. Does in vitro susceptibility testing predict clinical outcomes in bacterial keratitis? -Results from the steroids for corneal ulcers trial (SCUT) pilot study. Invest Ophthalmol Vis Sci. 2007;48:4277.
Acknowledgements
This research/work was supported by the NIHR Cambridge Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND Drafting the work or revising it critically for important intellectual content; AND Final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moledina, M., Roberts, H.W., Mukherjee, A. et al. Analysis of microbial keratitis incidence, isolates and in-vitro antimicrobial susceptibility in the East of England: a 6-year study. Eye 37, 2716–2722 (2023). https://doi.org/10.1038/s41433-023-02404-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02404-3
This article is cited by
-
A comparison of antimicrobial regimen outcomes and antibiogram development in microbial keratitis: a prospective cohort study in Alexandria, Egypt
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Microbiological profile of infectious keratitis in the Newcastle and Gateshead region: a 10-year analysis
Eye (2023)