Abstract
Background
We aim to characterise the ophthalmic findings and retinal vasculature changes in patients with WS, and to analyse the correlation between ophthalmic manifestations and the associated systemic diseases.
Methods
This retrospective case-control study included 27 WS patients and 28 age-matched healthy participants. Stellate pattern of iris, central macular thickness (CMT), foveal width, retinal vessel diameter, superficial vascular density (SVD) of macula and foveal avascular zone (FAZ) were compared between WS patients and healthy participants.
Results
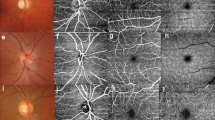
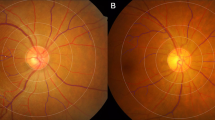
Twenty-five patients (93%) had the classic stellate iris presentation. Compared with healthy controls, WS patients had decreased CMT, increased foveal width and a lower SVD of macula (all P < 0.001). Significantly decreased mean retinal arterial (117.9 ± 9.9 µm vs. 133.0 ± 6.7 µm in WS and controls, respectively; p < 0.001) and venous (158.9 ± 11.2 µm vs. 174.0 ± 8.0 µm in WS and controls, respectively; p < 0.001) outer diameters, as well as mean arterial wall thickness (11.2 ± 1.3 µm vs. 12.2 ± 0.8 µm in WS and controls, respectively; p < 0.01) were found in WS. Stellate iris grading was significantly associated with CMT, foveal width, retinal vessel diameter (all p < 0.05), and a significant increase in the odds of having hypertension (Odds ratio (OR), 5.63; P < 0.05). The severity of stellate iris in WS seemed to have the trend of increasing risk of having pulmonary stenosis, tricuspid regurgitation and mitral regurgitation.
Conclusions
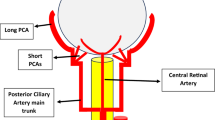
This study provides the first in vivo evidence reflecting current knowledge on vessel morphology in WS patients that deficient circumferential growth is the predominant pathophysiologic changes resulting from elastin deficiency. The ophthalmic characteristics may serve as a complementary tool to diagnose and follow-up patients suffering from WS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available upon reasonable request.
References
Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol. 2002;17:269–71.
Korenberg JR, Chen XN, Hirota H, Lai Z, Bellugi U, Burian D, et al. VI. Genome structure and cognitive map of Williams syndrome. J Cogn Neurosci. 2000;12:89–107.
Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362:239–52.
Collins RT, Kaplan P, Somes GW, Rome JJ. Long-Term Outcomes of Patients With Cardiovascular Abnormalities and Williams Syndrome. Am J Cardiol. 2010;105:874–8.
Collins RTI. Cardiovascular disease in Williams syndrome. Curr Opin Pediatrics 2018;30:609–15.
Leung DY, Glagov S, Mathews MB. Elastin and collagen accumulation in rabbit ascending aorta and pulmonary trunk during postnatal growth. Correlation of cellular synthetic response with medial tension. Circ Res. 1977;41:316–23.
Keating MT. Genetic Approaches to Cardiovascular Disease. Circulation 1995;92:142–7.
Parks WC, Pierce RA, Lee KA, Mecham RP. Elastin. In: Bittar EE, editor. Advances in Molecular and Cell Biology. Vol 6. Extracellular Matrix. Elsevier; Volume 6; 1993. p. 133–181.
Holmström G, Almond G, Temple K, Taylor D, Baraitser M. The iris in Williams syndrome. Arch Dis Child. 1990;65:987–9.
Winter M, Pankau R, Amm M, Gosch A, Wessel A. The spectrum of ocular features in the Williams-Beuren syndrome. Clin Genet. 1996;49:28–31.
Greenberg F, Lewis RA. The Williams syndrome. Spectrum and significance of ocular features. Ophthalmology 1988;95:1608–12.
Weber Sarah LP, Souza Rodrigo B, Ribeiro Lorena G, Tavares Marcela F, Goldchmit M. Williams Syndrome: Ophthalmological Examination and Review of Systemic Manifestations. J Pediatr Ophthalmol Strabismus. 2014;51:209–13.
Popovic N, Radunovic M, Badnjar J, Popovic T. Fractal dimension and lacunarity analysis of retinal microvascular morphology in hypertension and diabetes. Microvascular Res. 2018;118:36–43.
Cabrera DeBuc D, Somfai GM, Koller A. Retinal microvascular network alterations: potential biomarkers of cerebrovascular and neural diseases. Am J Physiol-Heart Circulatory Physiol. 2016;312:H201–H212.
Cheung CY, Thomas GN, Tay W, Ikram MK, Hsu W, Lee ML, et al. Retinal Vascular Fractal Dimension and Its Relationship With Cardiovascular and Ocular Risk Factors. Am J Ophthalmol. 2012;154:663–674.e1.
Genetics C on. Health Care Supervision for Children With Williams Syndrome. Pediatrics 2001;107:1192–204.
Ctori I, Huntjens B. Repeatability of Foveal Measurements Using Spectralis Optical Coherence Tomography Segmentation Software. PLoS One. 2015;10:e0129005.
Tick S, Rossant F, Ghorbel I, Gaudric A, Sahel J-A, Chaumet-Riffaud P, et al. Foveal shape and structure in a normal population. Investig Ophthalmol Vis Sci. 2011;52:5105–10.
Muraoka Y, Tsujikawa A, Kumagai K, Akiba M, Ogino K, Murakami T, et al. Age- and hypertension-dependent changes in retinal vessel diameter and wall thickness: an optical coherence tomography study. Am J Ophthalmol. 2013;156:706–14.
Alten F, Motte J, Ewering C, Osada N, Clemens CR, Kadas EM, et al. Multimodal Retinal Vessel Analysis in CADASIL Patients. PLOS ONE 2014;9:e112311.
Williams JCP, Barratt-Boyes BG, Lowe JB. Supravalvular Aortic Stenosis. Circulation 1961;24:1311–8.
Castelo-Branco M, Mendes M, Sebastião AR, Reis A, Soares M, Saraiva J, et al. Visual phenotype in Williams-Beuren syndrome challenges magnocellular theories explaining human neurodevelopmental visual cortical disorders. J Clin Investig. 2007;117:3720–9.
Chen K, Weiland JD. Discovery of retinal elastin and its possible role in age-related macular degeneration. Ann Biomed Eng. 2014;42:678–84.
Milani P, Montesano G, Rossetti L, Bergamini F, Pece A. Vessel density, retinal thickness, and choriocapillaris vascular flow in myopic eyes on OCT angiography. Graefes Arch Clin Exp Ophthalmol. 2018;256:1419–27.
Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, et al. A critical role for elastin signaling in vascular morphogenesis and disease. Development 2003;130:411–23.
Urbán Z, Riazi S, Seidl TL, Katahira J, Smoot LB, Chitayat D, et al. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am J Hum Genet. 2002;71:30–44.
Jiao Y, Li G, Korneva A, Caulk AW, Qin L, Bersi MR, et al. Deficient Circumferential Growth Is the Primary Determinant of Aortic Obstruction Attributable to Partial Elastin Deficiency. Arteriosclerosis Thrombosis Vasc Biol. 2017;37:930–41.
Lee WH, Park J-H, Won Y, Lee M-W, Shin Y-I, Jo Y-J, et al. Retinal Microvascular Change in Hypertension as measured by Optical Coherence Tomography Angiography. Sci Rep. 2019;9:156.
Majeed A, Marwick B, Yu H, Fadavi H, Tavakoli M. Ophthalmic Biomarkers for Alzheimer’s Disease: A Review. Front Aging Neurosci. 2021;13:720167.
Wessel A, Motz R, Pankau R, Bürsch JH. Arterial hypertension and blood pressure profile in patients with Williams-Beuren syndrome. Z Kardiol. 1997;86:251–7.
Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, et al. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Investig. 2003;112:1419–28.
Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54:74–95.
Funding
This study is supported by Taipei Veterans General Hospital (grant no. VGH V110C-052, V111C-099) and Ministry of Science and Technology of Taiwan (grant no. MOST 110-2314-B-075-054-MY3, MOST 111-2324-B-075-42).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: T-CY, H-CC, D-MN, A-GW (II) Administrative support: H-YL, SCC, H-YY, J-YY. (III) Provision of study materials or patients: H-CC, A-GW. (IV) Collection and assembly of data: T-CY. (V) Data analysis and interpretation: T-CY, H-CC, A-GW. (VI) Paper writing: T-CY, H-CC. (VII) Final approval of paper: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yeh, TC., Cheng, HC., Li, HY. et al. Ophthalmic characteristics and retinal vasculature changes in Williams syndrome, and its association with systemic diseases. Eye 37, 2265–2271 (2023). https://doi.org/10.1038/s41433-022-02328-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02328-4