Abstract
Introduction
COVID-19 has impacted ophthalmic care delivery, with many units closed and several ophthalmologists catching COVID-19. Understanding droplet spread in clinical and training settings is paramount in maintaining productivity, while keeping patients and practitioners safe.
Objectives
We aimed to assess the effectiveness of a breath-guard and a face mask in reducing droplet spread within an eye clinic.
Methods
We performed a randomised trial of droplet spread using a fluorescein-based cough model to assess the efficacy of a ‘breath-guard’ and ‘face-mask’ to prevent the spread of droplets. The ‘cough’ spray was collected on calibrated paper targets. The sheets were photographed under blue light, with an orange filter on the camera; the position and size of the spots was measured with software originally developed for astronomy.
We performed 44 randomised coughs; 22 controls with no breath-guard or face-mask, 11 using breath-guard only and 11 with combined breath-guard and face-mask. We compared both the number of droplets detected and the area of drops on paper targets.
Results
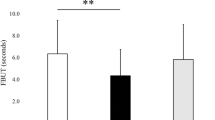
The average number of droplets in the controls was 19,430 (SE 2691), the breath-guard group 80 (SE 19) droplets (P < 0.001); in the combined In the group the count was 5 (SE 2), a significant drop from shield only (P = 0.008). The mean areas of each target covered by spots for each group were 5.7 ± 0.857% (95% CI), 0.004 ± 0.000104% (95% CI) and 0.001 ± 0.0000627% (95% CI) respectively.
Conclusion
These results show that the breath-guard alone reduced the droplet count by 99.93%. Combining the breath-guard with a face-mask reduced the droplet count by over 99.98%. Breath-guards are widely used in clinics and this trial demonstrates that breath-guards with face-masks effectively block droplet spray.
Similar content being viewed by others
Introduction
Transmission of COVID-19 within ophthalmology clinics is recognised as a significant danger to both patients and staff members. The sustained close proximity of patients to healthcare workers in enclosed (and sometimes poorly ventilated) ophthalmology clinics leads to high levels of droplet and aerosol contamination [1]. This is reflected in a high rate of cross infection and the death of several ophthalmologists during the pandemic [2,3,4].
Evidence suggests that person-to-person transmission occurs through droplets, or aerosols, of infected saliva and respiratory secretions when a person coughs, sneezes or talks [5,6,7]. Jones et al. [8] had previously reported that larger respiratory droplets, such as those expelled for a cough, would fall quicker than smaller aerosolized particles from speech due to gravitational forces. However, some now consider droplets up to 100 μm as aerosols, and others have shown that a cough can propel droplets of these sizes for over 8 m [9, 10]. A recent trial by our group, using a cough model, has shown that droplets regularly travel further than 2 m even within a laminar flow operating theatre [11].
The use of ophthalmic equipment, such as the slit lamp biomicroscope or direct ophthalmoscope, require close working distances. Common transmission routes for COVID-19 are from droplets or aerosols, via the eye [12,13,14,15] or from fomite spread [16]. Ophthalmologists and other eye care health professionals, who undertake face-to-face patient clinical examinations, are therefore at a heightened risk of transmission of the COVID-19 virus [17].
Patient infection risk may also be great, and it should be recognised that 25–30% of all COVID-19 deaths are thought to have originated from health care workers, many of whom are asymptomatic [18, 19]. This indicates the need for precautionary measures within ophthalmic departments, who treat mainly elderly patients, and other eye care practices to minimise risk of transmission whilst undertaking ophthalmic investigations [20].
To mitigate these risks, patients have been encouraged to wear face-masks and health care workers have worn PPE. Other methods include increased ventilation and physical barriers or ‘breath-guards’ to prevent a direct spray between patient and staff [21].
The Portsmouth group has developed a number of methods for generating a cough model, and detecting the spray of droplets in different clinical situations. We stain droplets with fluorescein, and use forensic imaging techniques to photograph the droplets. Droplets were counted using image analysis techniques in the Institute of Cosmology and Gravitation.
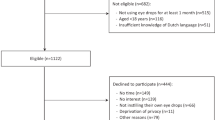
We performed a randomised controlled trial of a control group with no shield vs ‘breath-guard’ vs ‘breath-guard and face-mask’ to measure the effectiveness of the breath-guard on droplet contact with the clinician. In the remainder of this paper, we refer to the ‘breath-guard and face-mask group’ as simply the ‘breath-guard group’.
Methods
We used a cough model, taped to a slit lamp in the University of Portsmouth Eye Clinic. The cough model uses an Ambu bag connected to 40 cm of 30 mm endotracheal tubing. We calibrated this to give a FEV1 between 290 and 370 l/mm, similar to a human cough. To produce a ‘dry’ cough we used 0.5 ml of normal saline, stained this with 1 mg/100 ml fluorescein (Fig. 1).
We used a randomised controlled trial method, random numbers were used to determine whether the breath-guard, breath-guard with a face-mask or control. The breath-guard we used was 300 mm high and 350 mm wide and was attached to the eye pieces of the slit lamp (Fig. 2).
We placed a target of four A4 printed sheets on a board at 40 cm (the standard position of the observer eyepieces) from the cough model, behind the slit lamp and triggered the ‘cough’. The targets were imaged under UV light in a dark room, using a blue blocking filter (Tiffen, New York, Orange 16). Sets of images were taken with the control (22), the breath-guard (11) with the breath-guard and face-mask (11).
The images were first de-warped to correct the camera angle, and a source detection algorithm, Source Extractor [22], was then used to detect droplet ‘spots’ on the targets. The algorithm identified spots that were an area of 5 pixels or larger, which corresponds to a physical area of approximately 40 μm2 (Fig. 3). We compared the total numbers of spots for each group, as well as the total area of splatter on each target.
This research was submitted to the University of Portsmouth Ethics screening tool ref: 10247.
Results
We detected 19,430 (SE 2,691) droplets in the control group; 80 (SE 19) in the breath-guard (p < 0.001), and 5 (SE 2) in the face-mask group (P = 0.008). (Fig. 4). The average area of each target covered by the droplets in the control group was 5.7 ± 0.857% (95% CI), 0.004 ± 0.000104% (95% CI) in the breath-guard group (95% CI 0.02, 0.001), and 0.001 ± 0.0000627% (95% CI).
Using the mean area of the targets covered in spots as a measure, there was a 99.93% reduction in droplet spread with breath-guard and a 99.98% with the face-mask and breath-guard group. We also noted fluorescein splattered on the floor and on the researchers, this was invisible to the human eye but illumination under UV light revealed the spots within the environment.
Discussion
The results show the sensitivity of this technique in detecting droplets, and the number of droplets that a single cough can generate, with the potential of COVID-19 infection within an eye clinic. This has been suggested in other clinical environments [9], however this is the first time it has been demonstrated within an eye clinic.
The breath-guard effectively reduced the spread of droplets from the cough model, this effect was enhanced using a face-mask. We found a 99.93% fall in the area of spots detected with a breath-guard and 99.98% reduction with the face-mask as well as the breath-guard. However, some drops still escaped and as only one droplet may be sufficient to transmit COVID-19 other measures to prevent droplet transmission are clearly still vital. These results are consistent with the droplet spread found in other healthcare environments. A physical barrier may be key to prevent droplet spread. This is clearly an important feature of safety within the ophthalmology clinic for slit lamp users but for other equipment as well (such as autorefractors and biometry).
However, the breath-guards are not protective against the smallest droplets such as aerosols [23], these will remain airborne for many minutes, and may affect the physician as well as the patient and subsequent patients. Good ventilation is important as well and the air turnover is recommended at 10 cycles per minute [24]. This is a great change for most eye clinics, many of which are situated in unventilated areas of the hospital.
One last consideration was that the fluorescein actively stained fluid that was detected on the floor, chair, swabs and on the instruments. This was of considerable surprise to the ophthalmologist. Use of fluorescein in this way may be a useful clinical test for fomite transfer [25] within an eye clinic and to monitor cleaning [26].
The limitations of this trial were that it was not possible to accurately measure the aerosol release and as these play a vital role in the propagation of disease [27] it was not possible to fully understand the effectiveness of the breath-guard. It may also be useful to assess other sizes of breath-guard and types of face-masks [28, 29]. Other areas of interest would be the effect of smaller cough volumes, the effect of face-masks [30] and the effect of ventilation / air filtration equipment.
Given the relative size of the droplets the volume of virus carried in these is large and protection against droplets plays an important role within the eye clinic.
Conclusion
Larger breath-guards significantly reduced the droplets spread, with the addition of face-masks very few droplets spread from the patient. Use of face-masks and breath-guards play an important role in reducing the viral load spread between the clinician and the patient. Smaller guards may not be as effective. It would be useful to test the model on patient volunteers and to assess the effect of aerosols and ventilation on respiratory virus transmission, within eye clinics.
Summary
What was known before
-
Ophthalmologist and optometrist were at risk of catching COVID-19.
What this study adds
-
A breath-guard reduces the spread of droplets by 99.8% within eye clinics. The fluorescein and Astrophysics software to analyse droplet spread in a clinical environment was described.
References
Sadhu S, Agrawal R, Pyare R, Pavesio C, Zierhut M, Khatri A, et al. COVID-19: limiting the risks for eye care professionals. Ocul Immunol Inflamm. 2020;28:714–20.
Romano MR, Montericcio A, Montalbano C, Raimondi R, Allegrini D, Ricciardelli G, et al. Facing COVID-19 in Ophthalmology Department. Curr Eye Res. 2020;45:653–8.
Nair AA. Staring death in the eyes: fighting on the COVID frontline as an ophthalmologist. Am J Ophthalmol. 2020;220:A15–6.
Kursumovic E, Lennane S, Cook TM. Deaths in healthcare workers due to COVID-19: the need for robust data and analysis. Anaesthesia. 2020;75:989–92.
WHO. Modes of transmission of virus causing COVID-19: implications for IPC precautions and recommendations. 2020. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
Johnson GR, Morawska L, Ristovski ZD, Hargreaves M, Mengersen K, Chao CYH, et al. Modality of human expired aerosol size distributions. J Aerosol Sci. 2011;42:839–51.
Anfinrud P, Stadnytskyi V, Bax CB, Adriaan Bax A. Visualizing speech-generated oral fluid droplets with laser light scattering. N Engl J Med. 2020;382:2061–3.
Jones NR, Qureshi ZU, Temple RJ, Larwood LPJ, Greenhalgh T, Bourouiba L. Two metres or one: what is the evidence for physical distancing in covid-19? BMJ. 2020;370:m3223.
Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323:1837–8.
Morawska L, Cao J. Airborne transmission of SARS-CoV-2. The world should face the reality. Environ Int. 2020;139:105730.
Newsom RB, Amara A, Hicks A, Quint M, Pattison C, Bzdek BR, et al. Comparison of droplet spread in standard and laminar flow operating theatres: SPRAY study group. J Hosp Infect. 2021;110:194–200.
Wang L, Wang Y, Ye D, Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int J Antimicrob Agents. 2020;55:105948.
Qing H, Li Z, Yang Z, Shi M, Huang Z, Song J, et al. The possibility of COVID-19 transmission from eye to nose. Acta Ophthalmol. 2020;98:e388.
Belser JA, Lash RR, Garg S, Tumpey TM, Maines TR. The eyes have it: influenza virus infection beyond the respiratory tract. Lancet Infect Dis. 2018;18:e220–7.
Deng W, Bao L, Gao H, Z Xiang, Y Qu, Z Song, et al. Rhesus macaques can be effectively infected with SARS-CoV-2 via ocular conjunctival route. 2020. https://www.biorxiv.org/content/10.1101/2020.03.13.990036v1.
Xie HT, Jiang SY, Xu KK, Liu X, Xu B, Wang L, et al. SARS-CoV-2 in the ocular surface of COVID-19 patients. Eye Vis. 2020;7:23.
Ong SWX, Tan KY, Chia PY, Lee TH, Ng OT, Wong SUY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–2.
Heneghan C, Howdon D, Oke J, Jefferson T. The Ongoing Problem of UK Hospital Acquired Infections. 2020. https://www.cebm.net/covid-19/the-ongoing-problem-of-hospital-acquired-infections-across-the-uk.
Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl J Med. 2020;382:2081–90. https://doi.org/10.1056/NEJMoa2008457.
Wan Kelvin H, Lin Timothy PH, Ko C-N, Lam Dennis SC. Impact of COVID-19 on ophthalmology and future practice of medicine. Asia-Pac J Ophthalmol. 2020;9:279–80.
Lam DSC, Wong RLM, Lai KHW, Chung-Nga K, Hiu YL, Wing Lee VY, et al. COVID-19: special precautions in ophthalmic practice and FAQs on personal protection and mask selection. Asia Pac J Ophthalmol. 2020;9:67–77.
Bertin E, Arnouts S. SExtractor: software for source extraction. Astron Astrophys Suppl Ser. 1996;117:393–404.
Walker JS, Archer J, Gregson FAK, Michel SES, Bzdek BR, Reid JP. Accurate representations of the microphysical processes occurring during the transport of exhaled aerosols and droplets. ACS Cent. Sci. 2021. https://doi.org/10.1021/acscentsci.0c01522.
World Health Organization. Transmission of SARS-CoV-2: implications for infection preventive precautions: scientific brief, 9 July 2020. World Health Organization; 2020. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions.
Santarpia JL, Rivera DN, Herrera VL, Morwitzer MJ, Creager HM, Santarpia GW, et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care [published correction appears in Sci Rep. 2020 Aug12;10(1):13892]. Sci Rep. 2020;10:12732.
Doremalen N, Bushmaker T, Morris DH, Myndi GH, Amandine G, Brandi NW, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–7.
Bischoff WE, Swett K, Leng I, Peters TR. Exposure to influenza virus aerosols during routine patient care. J Infect Dis. 2013;207:1037–46.
Leung NHL, Chu DKW, Shiu EYC, Kwok-Hung C, James JM, Benien JPH, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks [published correction appears in Nat Med. 2020 May 27;:]. Nat Med. 2020;26:676–80.
Bartoszko JJ, Farooqi MAM, Alhazzani W, Mark L. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: A systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses. 2020;14:365–73.
Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–87.
Acknowledgements
With thanks to Dr. Coleman Krawczyk for software development, Alex Sale for photography and Daniel Stride for the use of the University of Portsmouth Eye Clinic facilities.
Funding
This work was supported by an impact accelerator grant from the Science and Technologies Facilities Council. AA is supported by a Royal Society Wolfson Fellowship.
Author information
Authors and Affiliations
Contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. RN: Developing the technique, design of trial, capturing data, document preparation and submission. CP: Photography, image analysis, statistical analysis, document review. AL: Photography, data capture, image analysis, statistical analysis, document review. PR: Developing the technique, design of trial, capturing data, document review. MC: Development of the cough model and document review. AA: Imaging, image analysis, statistical analysis, document review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Newsom, R., Pattison, C., Lundgren, A. et al. Comparison of breath-guards and face-masks on droplet spread in eye clinics. Eye 37, 2135–2138 (2023). https://doi.org/10.1038/s41433-022-02308-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02308-8