Abstract
Background
Voretigene neparvovec (VN) is a gene therapeutic agent for treatment of retinal dystrophies caused by bi-allelic RPE65 mutations. We illustrate, both the benefits and pitfalls associated with ocular gene therapy in the same patient.
Methods
Two eyes of one patient with bi-allelic RPE65 mutations have been treated with VN. The clinical examinations included visual acuity (VA, in normal and low luminance), colour vision, contrast sensitivity, International Society for Clinical Electrophysiology of Vision (ISCEV) standard retinal electrophysiology and dark-adapted full-field stimulus threshold (FST), Goldmann VF analysis and imaging studies, including optical coherence tomography (OCT) and autofluorescence. These were performed at baseline, 2-weeks, 3 and 6-months, 1 and 2-years follow-up.
Results
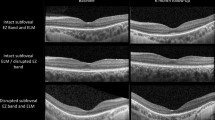
The first eye showed improvement in rod photoreceptor function with increased peripheral and low luminance vision (baseline VA: 0.9 logMAR and 2-years post-operative VA: 0.7 logMAR). The second eye, whilst showing increased light sensitivity, suffered a drop in central vision (at 2-weeks) with loss of foveal photoreceptors as shown by the loss of ellipsoid zone on OCT scan (baseline VA: 0.6, 2-year post-operative VA: 1.2). FST improvements were maintained in both eyes indicating a sustained efficacy of VN with little waning of its effect.
Conclusions
We present a previously unreported adverse complication of subretinal VN therapy in bi-allelic RPE65, indicating a probable immune response in treatment of the second eye, resulting in loss of foveal photoreceptors. This case-series highlights the potential and pitfalls of retinal gene therapy in the same patient. The immune responses of the body to a ‘foreign vector’, remains a challenge.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw data are available upon reasonable request.
References
RetNet. Summaries of genes and loci causing retinal diseases. 2016. https://sph.uth.edu/retnet/sum-dis.htm. Accessed 10 Apr 2017.
Thompson DA, Gyürüs P, Fleischer LL, Bingham EL, McHenry CL, Apfelstedt-Sylla E, et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Investig Ophthalmol Vis Sci. 2000;41:4293–9.
den Hollander AI, Roepman R, Koenekoop RK, Cremers FPM. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retinal Eye Res. 2008;27:391–419.
Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retinal Eye Res. 2010;29:398–427.
Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–605.
Bennett J, Wellman J, Marshall KA, McCague S, Ashtari M, DiStefano-Pappas J, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388:661–72.
Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–60.
National Institute for Health and Care Excellence; Voretigene neparvovec for treating inherited retinal dystrophies caused by RPE65 gene mutations. National Institute for Health and Care Excellence. 2019.
Friedmann T, Roblin R. Gene therapy for human genetic disease? Science. 1972;175:949–55.
Maguire AM, Russell S, Wellman JA, Chung DC, Yu ZF, Tillman A, et al. Efficacy, safety, and durability of voretigene neparvovec-rzyl in RPE65 mutation–associated inherited retinal dystrophy: results of phase 1 and 3 trials. Ophthalmology. 2019;126:1273–85.
Deng C, Zhao PY, Branham K, Schlegel D, Fahim AT, Jayasundera TK, et al. Real-world outcomes of voretigene neparvovec treatment in pediatric patients with RPE65-associated Leber congenital amaurosis. Graefe’s Arch Clin Exp Ophthalmol. 2022;260:1543–50.
Chandler LC, McClements ME, Yusuf IH, Martinez-Fernandez de la Camara C, MacLaren RE, Xue K. Characterizing the cellular immune response to subretinal AAV gene therapy in the murine retina. Mol Ther Methods Clin Dev. 2021;22:52–65.
Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye. 2002;16:411–21. https://www.nature.com/articles/6700197. Accessed 15 May 2022.
Guerin CJ, Anderson DH, Fariss RN, Fisher SK. Retinal reattachment of the primate macula. Photoreceptor recovery after short-term detachment. Investig Ophthalmol Vis Sci. 1989;30:1708–25.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
As this was a retrospective anonymized study, as per our local protocol from our Clinical Effectiveness Department, and as per national guidelines from the National Code of Clinical Research, and the Health Research Authority (HRA), this study has ethical approval exemption and no patient consent was required for participation. All procedures were completed prior to the design of this study. Patients were diagnosed and treated according to local guidelines and agreements and written consent from patients was acquired prior to all procedures as clinically indicated. This study does not report on the use of new or experimental protocols.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jalil, A., Ivanova, T., Moussa, G. et al. Retinal gene therapy in RPE-65 gene mediated inherited retinal dystrophy. Eye 37, 1874–1877 (2023). https://doi.org/10.1038/s41433-022-02262-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02262-5
This article is cited by
-
Full-field stimulus threshold testing: a scoping review of current practice
Eye (2024)
-
Voretigene-neparvovec
Reactions Weekly (2023)