Abstract
Aim
This study uses OCT imaging to examine morphological changes at the chorioretinal interface of the choroidal melanoma biopsy site over time.
Setting
Liverpool Ocular Oncology Centre (LOOC), St. Paul’s Eye Unit, Royal Liverpool University Hospital, Liverpool, United Kingdom.
Methodology
Retrospective analysis of all patients who underwent 25G vitrector-assisted transretinal biopsy for choroidal melanoma between 1st Jan 2017 and 31st Dec 2019. The biopsy procedure does not involve full vitrectomy, treatment of the retinal hole or tamponade. Patients routinely undergo OCT imaging over the tumour site at each follow up. Cases required a minimum of a baseline OCT and two consecutive post-operative OCT scans of adequate quality, overlying the biopsy site. All images were reviewed and graded by an ophthalmic specialist.
Results
Fifty-one patients met the criteria for inclusion. OCT analysis identified 2 characteristic morphologies following transretinal biopsy –a choroidal plugging of biopsy site (47.0%), or a flat retinal hole (53.0%). Choroidal plugging morphologies tend to remain unchanged over time, whereas flat holes demonstrate greater variability and would more commonly seal over with atrophic retina (Fisher Exact Value = <0.01). 60.8% demonstrated vitreous plugging of the biopsy site.
Conclusion
OCT analysis has identified key morphological changes following transretinal choroidal melanoma biopsy. The morphologies identified may provide dynamic protective effects against vitreous flow into the subretinal space, accounting for low rates of retinal detachment despite the presence of an untreated iatrogenic retinal hole following transretinal biopsy.
Similar content being viewed by others
Introduction
Intraocular biopsies have evolved as an important part in the management of choroidal melanoma. Histopathological and cytogenetic analysis of biopsy specimens allow for diagnosis, determination of tumour characteristics and likelihood of metastasis [1]. Surgical approaches vary significantly, depending on the tissue involved, size and location of the lesion, and surgeons’ preferences.
A transretinal approach allows better visualisation and access to more posteriorly located tumours, whereas a trans-scleral approach may be preferred for lesions located more anteriorly. Previous studies report yields between 85 and 99% for histopathological analysis, with rates of significant complications, such as vitreous haemorrhage requiring surgical intervention, retinal detachment and tumour seeding, ranging between 0 and 3.6% [1,2,3,4,5,6]. There is no consensus regarding the optimal surgical technique for transretinal biopsies of tumours at the posterior pole. Published approaches vary significantly, including fine needle aspiration biopsy [7,8,9], a transretinal technique using a vitreous cutter [10], or complete vitrectomies with endotamponade, retinopexy, and cryopexy to the ports [5, 11,12,13].
At the Liverpool Ocular Oncology Centre (LOOC), diagnostic and prognostic tumour biopsy is discussed with every uveal melanoma patient. With informed consent, transretinal tumour biopsy is performed using a vitreous cutter, through a standard 25-gauge 3-port vitrectomy setup. The technique has previously been described in detail [10]. Importantly, this technique is performed without vitrectomy, retinopexy, tamponade or cryotherapy of sclerotomies, following Ruthenium-106 brachytherapy or proton beam radiotherapy. The ‘no vitrectomy, no retinopexy, no tamponade’ approach is controversial, as conventional thinking suggests that a retinal defect that is not sealed by retinopexy and not supported by endotamponade is associated with significant risks, i.e., postoperative retinal detachment or tumour seeding. However, a significant number of such events following this procedure have not been seen [2,3,4].
Spectral-domain ocular coherence tomography (OCT) allows for accurate identification of retinal layers, the retinal pigment epithelium (RPE) and choroid, and for changes in these layers to be noted even following radiotherapy for choroidal melanoma [14].
Aim
To ascertain why untreated full-thickness retinal defects following tumour biopsies do not lead to a greater number of retinal detachments, this study was performed to examine the retinal changes at the transretinal biopsy site in greater detail with OCT and better understand iatrogenic structural changes associated with this procedure.
Methodology
A retrospective review of all patients who underwent transretinal biopsy for choroidal melanoma at the LOOC between 1st Jan 2017 and 31st Dec 2019 was undertaken. Patients had received treatment with Ruthenium-106 plaque or proton beam radiotherapy prior to biopsy. The surgical details of these treatments have previously been described [14]. Patient baseline characteristics, ophthalmic examination and OCT images were obtained from electronic medical records. Patients routinely undergo a complete slit-lamp examination and OCT imaging of the posterior pole using the Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) with two 19-line volume scans with a setting of 9 automatic real time centred over the tumour area and fovea at each visit.
Inclusion criteria include having a baseline and a minimum of two consecutive postoperative OCT scans showing the biopsy site at different time points, with an adequate OCT image quality as judged by an ophthalmic specialist. OCT analysis included assessment of posterior hyaloid face morphology, neurosensory retinal layers, RPE, choroidal layer, and presence of retinal fluid. Any atypical, non-categorical features were also noted. All images were reviewed and graded by an ophthalmic specialist, and uncertainties were referred to an independent second ophthalmic specialist for opinion.
Follow-up examinations were performed at varying timepoints, depending on individual patient characteristics including tumour size and location, associated ocular complications, distance of travel to the LOOC, and availability of appropriate follow-up examinations closer to the patients’ place of residence. Routinely, follow-up examinations are scheduled 3 to 6 months after the radiotherapy treatment and biopsy, and then 6 to 12-monthly subsequently. Follow-up imaging data were obtained between date of diagnosis and 1st October 2020.
Transretinal biopsy technique
All surgeries were performed, using a standard operating microscope (Haag-Streit, Zug, Switzerland) with a mounted non-contact retinal viewing lens (Eibos, Haag-Streit, Zug, Switzerland). One of three consultant vitreo-retinal surgeons, or a trained, supervised vitreo-retinal fellow, would perform the surgery using the standardised technique as described. Transretinal biopsies were performed under local or general anaesthesia with a 25-gauge vitreous cutter, in a 3-port sutureless vitrectomy set-up (Alcon Constellation, Geneva, Switzerland). This has been described in further detail previously [10]. In summary, an infusion line is connected to one of the trocars with the other two trocars being used for endo-illumination and the vitreous cutter. A 3-way tap is inserted into the standard suction line leading to the vitrectomy machine and is used to backflush the specimen once retrieved. The cutter is advanced through the vitreous and then pushed through the retina into the tumour tissue, ideally over the apex of the tumour and avoiding major retinal blood vessels. The cutter is advanced as close to the sclera as possible, the cutter is activated (cut rate 1000 cpm, vacuum 600 mmHg) and the biopsy is performed for approximately one minute, or until biopsy material becomes visible in the suction tubing. The cutter is then withdrawn, and the biopsy specimen is flushed back through the cutter into a specimen tube with balanced salt solution injected through the 3-way tap. The tube is inspected for visible biopsy material and the cutter is re-introduced through the retinal defect for a repeat biopsy if the specimen is deemed insufficient. The trocars are removed and, after ascertaining the absence of a vitreous wick, the conjunctiva is massaged over the trocar site; the ports are not routinely sutured. Importantly, biopsies were performed without vitrectomy, retinopexy of the retinal defect, endotamponade or cryotherapy of sclerotomies.
All biopsies are performed after radiation therapy. Typically, it is between 0 and 4 weeks following proton beam radiotherapy, and at around 4 weeks following plaque radiotherapy. The timing is primarily guided by logistical factors including surgeon, operating theatre slot and patient availability and patient availability, as well as patient recovery following radiotherapy.
Informed patient consent was obtained for surgical procedures as well as collection and analysis of all data. Ethics committee approval was not required for this retrospective data analysis. The study adheres to the tenets of the Declaration of Helsinki.
Results
Fifty-one patients met the OCT requirement criteria (see methodology). The reasons for excluding patients from the study were insufficient number of OCT scans performed at LOOC, inadequate scan quality over the biopsy site and biopsy site outside the reach of routine OCT imaging. Of the 51 eyes; all had a baseline OCT, 18 had an OCT within 3 months of biopsy, 39 had an OCT between 3 and 6 months, 40 patients at 12 months (±6 months), 22 at 24 months (±6 months) and 6 at 36 months (±6 months).
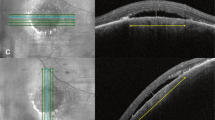
Posterior vitreous detachment (PVD) over the tumour and in areas adjacent to the tumour was 14/51 patients (27.5%). It was noted that even if a PVD were present, often there would be contact between the posterior hyaloid face and retinal surface at areas of choroidal elevation.
Intraretinal fluid (IRF) was noted in 17/51 patients (33.3%), and sub-retinal fluid (SRF) in 42/51 patients (82.4%). Intra-retinal hyper-reflective foci (intra-retinal HRF) were noted in 49/51 patients (96.1%).
OCT within 6 months
At the 6-month interval, 39 OCTs were analysed.
PVD was noted in 11/39 patients—of which, 5 were new. Interestingly, 21/39 patients (53.8%) developed vitreous adherence at the biopsy site, whereby the posterior hyaloid face would track into the biopsy site (Fig. 1). This was observed on OCTs as early as day 1 and day 20 following the biopsy.
Upon analysis of biopsy site morphology, two distinct patterns were noted at the choroidal interface:
The ‘tumour plug’ morphology appears as a hyper-reflective plug within the area of absent retina at the biopsy site (Fig. 2). This is likely to be plugging by choroidal tumour tissue, however, may also consist of a blood clot formation soon after biopsy, or, later, formation of scarring at the biopsy site. Nineteen patients (48.7%) demonstrated this pattern. Three out of these 19 patients demonstrated hyper-reflectivity at the corresponding RPE layer. The tumour plug had a flat top in majority of cases, however, in two cases the plug appeared to be pulled vertically into the vitreous cavity (Fig. 3).
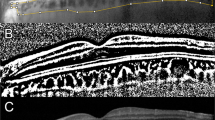
An open, retinal hole morphology was seen in the remaining 20 patients (51.3%). This was identified by absence of retina, with no tumour plugging. Often, the edges of the site tapered down into the adjacent excavated choroid. (Fig. 4a). Further assessment demonstrated that although initially an absence of retina was noted, at the 6-month point, 9/20 (45%) patients demonstrated development of tissue formation sealing over the biopsy site—likely to represent an overlying glial membrane.
a OCT series demonstrating progression in an ‘open, retinal hole’ morphology at the biopsy site. Note the eventual choroidal atrophy, as highlighted by the arrowhead. b OCT series demonstrating progression in an ‘open, retinal hole’ morphology with development of an overlying glial membrane, as highlighted by the arrowhead.
Three-year OCT progression
Having identified two distinct morphologies, OCT analysis was performed in the time intervals accordingly. Several factors were analysed over the 3-year period (Table 1).
In total, 47.0% of cases demonstrated a tumour plug. The tumour plug morphology tends to remain relatively unchanged over the 3-year period. The hyperreflective tumour plug remains consistent over the 3 years, with minimal evidence of a glial membrane developing over the biopsy site. Approximately two-thirds of cases with a tumour plug morphology develop vitreous adherence to the biopsy site. There is little change in the presence of fluid, and the presence of hyperreflective foci is variable.
In contrast, the, open, retinal hole morphology showed greater variability. Of note, a glial membrane extends and covers the biopsy site in a proportion of patients, typically between 6 and 12 months. Atrophy of tissue at the choroid layer may also be seen (Fig. 4b). These findings were not seen in the tumour plug morphology. Approximately two-thirds of open, retinal hole morphology demonstrates vitreous adherence at the biopsy site.
Key characteristic differences were noted between the two morphologies in several time points. At 6 months, absence of choroidal tissue at the biopsy site was more frequently seen in the open, retinal hole morphology, as compared to the tumour plug (Fisher Exact Value = 0.02, p < 0.05). Formation of a glial membrane, that would extend over the biopsy site is more commonly seen in the open, retinal hole morphology (Fisher Exact Value = <0.01, p < 0.01). Conversely, hyperreflective choroid was noted consistently in the tumour plug morphology, as compared to the open, retinal hole morphology (Fisher Exact Value = 0.02, p < 0.05).
At 12 months, a significant difference was noted in the presence or absence of retina overlying the biopsy site. A glial membrane extended over the open retinal hole morphology, whereas the tumour plug would have a persistent absence of the retinal layer (Fisher Exact Value = <0.01, p < 0.01).
Discussion
Transretinal tumour biopsies without vitrectomy, retinopexy and endotamponade has been previously shown to be a safe method for obtaining tumour material [2, 4]. This technique is commonly challenged, as conventional thinking of vitreoretinal experts is that a retinal break would require treatment with retinopexy and internal tamponade to avoid retinal detachment. Key complications published in previous publications tend to include retinal detachment, vitreous haemorrhage, and tumour recurrence—ranging between 0 and 3.6% [1,2,3, 5, 6]. Previous reporting of the technique performed in Liverpool Ocular Oncology suggests a vitreous haemorrhage rate of 1.9%, rhegmatogenous retinal detachment 0.7% and endophthalmitis rate 0.14% [4]. Previous studies assessing rates of rhegmatogenous retinal detachments secondary to iatrogenic holes in macular hole surgery demonstrated rates of retinal detachment of 0–3.1% following iatrogenic retinal breaks [15, 16]. It is worth noting that, despite macular hole surgeries having a vitrectomy, the rates of retinal detachment secondary to an iatrogenic hole are similar to those demonstrated by the trans-retinal biopsy without vitrectomy [4].
These trans-retinal biopsies are performed after radiation treatment, as mentioned previously. Theoretically, one would expect reduced risk of seeding following biopsy, although this has not been formally investigated. Alongside this, logistically, it is preferrable to commence treatment prior to the biopsy, as this would eliminate the potential for treatment delays. As many patients are not local to the region, personal reasons including transport, accommodation, and occupational leave, may influence their availability for biopsy, thus needlessly delaying treatment.
The changes occurring at the biopsy site, as identified by this study, may explain the low rates of retinal complications despite an untreated iatrogenic retinal hole. To the best of our knowledge, this is the first study to examine transretinal biopsy sites on OCT imaging in greater detail. OCT has commonly been used to aid in diagnosis and monitoring of choroidal melanoma, however, this study has shown its utility to extend into assessing the effects of choroidal biopsy on chorioretinal architecture.
Two distinct morphological patterns of the biopsy site following 25G transretinal choroidal sampling were identified. The tumour plug results in choroidal tumour tissue filling the iatrogenic hole, thus preventing fluid from entering the subretinal space. This tends to remain stable, with minimal changes over the 3-year follow up period. In a small proportion of patients, the tumour plug was pulled further into the vitreous cavity – these also remained stable, with no increased risk of complications. The open, retinal hole patients typically demonstrated tapering down of the retinal edges, with little evidence of intra- or subretinal fluid. Approximately 50% of these patients developed a glial membrane over the biopsy site, typically noted at the 6-month OCT, which would therefore eliminate any long-term risk of retinal detachment. Both morphologies identified would prevent liquified vitreous from entering the subretinal space and causing subsequent retinal detachment.
In addition, the theoretical protective factors from retinal detachment include the formation of a round hole with no associated traction, the buckling effect of the underlying tumour, the scarring effects of the chorioretinal adhesion from radiation treatment, and the presence of blood at the biopsy site acting as a plug/glue.
A study by Grewal et al. studied the use of 4D microscope-integrated OCT in transvitreal retinochoroidal biopsy. Interestingly, the authors also demonstrated formation a tumour plug morphology intra-operatively, as the vitreous cutter was withdrawn from the choroidal tumour tissue [17]. It is worth noting, their vitrector settings were slightly different (cut rate 300 cpm, vacuum 600 mmHg) to that used in LOOC. One would expect a lower cut rate and higher vacuum may pull up tumour tissue and plug the gap formed by the biopsy, however, this was beyond the scope of our study, as the vitrectomy settings were the same in all cases at LOOC. Further to this, Murray et al. describes SD-OCT morphology of biopsy site following transretinal fine needle aspiration biopsy. 3 cases were analysed with biopsy day 1 post-biopsy. All 3 demonstrated a small wound gape, similar to the open, retinal hole morphology but smaller [18]. No plug morphology was noted in any case. This gives weight to the suggestion that the pull force of the vitrector vacuum plays a role in formation of the tumour plug.
An area of discussion, not analysed in this paper, would be the role of tumour size and treatment response on the morphology of the biopsy site. As aforementioned, the buckling effect of the choroidal melanoma may play a role in preventing retinal detachment following an iatrogenic hole. Following radiation therapy and tumour shrinking, as the tumour flattens, this may hasten covering of the hole with retinal or glial tissue.
Avoiding the full pars plana vitrectomy and gas or oil tamponade results in reduced operative times, surgical costs, as well as improving patient comfort and procedure related morbidity.
Conclusion
This study provides pertinent information to the short and longer-term OCT changes overlying the site of the 25 G cutter acquired transretinal biopsies. These findings aid in explaining the rationale behind its safety profile. In previous reports, we have also demonstrated its efficacy in terms of yield for histopathological and cytogenetic analysis, even in smaller tumours [2], as well as those taken post radiotherapy [19]. We would therefore advocate this technique for safe and effective diagnostic and prognostic biopsy tissue sampling of choroidal tumours.
Summary
What was known before
-
Transretinal biopsies are key in diagnosis, characterising and prognostication of choroidal melanomas.
-
Although transretinal biopsy results in a full thickness defect, even without full vitrectomy, retinopexy, or tamponade, it is not associated with a significant retinal detachment risk.
What this study adds
-
Discovered key morphologies that explain why untreated retinal holes from transretinal biopsies do not typically result in retinal detachment.
-
Explains the structural changes of retinal hole sites, secondary to transretinal biopsy, over a 3 year period.
Data availability
Data utilised for this study are stored on a secure database accessible to treating physicians within the Liverpool Ocular Oncology Centre. Raw, anonymised data are available upon request.
References
Kim RS, Chevez-Barrios P, Divatia M, Bretana ME, Teh B, Schefler AC. Yield, techniques, and complications of transvitreal and transscleral biopsies in small uveal melanoma. JAMA Ophthalmol. 2018;136:482–8. https://doi.org/10.1001/JAMAOPHTHALMOL.2018.0561.
Angi M, Kalirai H, Taktak A, Hussain R, Groenewald C, Damato BE, et al. Prognostic biopsy of choroidal melanoma: an optimised surgical and laboratory approach. Br J Ophthalmol. 2017;101. https://doi.org/10.1136/bjophthalmol-2017-310361.
Grewal DS, Cummings TJ, Mruthyunjaya P. Outcomes of 27-Gauge vitrectomy-assisted choroidal and subretinal biopsy. Ophthalmic Surg Lasers Imaging Retina. 2017;48. https://doi.org/10.3928/23258160-20170428-07.
Grixti A, Angi M, Damato BE, Jmor F, Konstantinidis L, Groenewald C, et al. Vitreoretinal surgery for complications of choroidal tumor biopsy. Ophthalmology. 2014;121. https://doi.org/10.1016/j.ophtha.2014.06.029.
Reddy DM, Mason LB, Mason JO, Crosson JN, Yunker JJ. Vitrectomy and vitrector port needle biopsy of choroidal melanoma for gene expression profile testing immediately before brachytherapy. Ophthalmology. 2017;124. https://doi.org/10.1016/j.ophtha.2017.03.053.
Seider MI, Berry DE, Schefler AC, Materin M, Stinnett S, Mruthyunjaya P, et al. Multi-center analysis of intraocular biopsy technique and outcomes for uveal melanoma: ocular Oncology Study Consortium report 4. Graefe’s Arch Clin Exp Ophthalmol. 2020;258. https://doi.org/10.1007/s00417-019-04531-8.
Shields CL, Ganguly A, Bianciotto CG, Turaka K, Tavallali A, Shields JA. Prognosis of uveal melanoma in 500 cases using genetic testing of fine-needle aspiration biopsy specimens. Ophthalmology. 2011;118. https://doi.org/10.1016/j.ophtha.2010.05.023.
Shields CL, Materin MA, Teixeira L, Mashayekhi A, Ganguly A, Shields JA. Small choroidal melanoma with chromosome 3 monosomy on fine-needle aspiration biopsy. Ophthalmology. 2007;114. https://doi.org/10.1016/j.ophtha.2007.04.054.
Singh, AD, Medina, CA, Singh, N, Aronow, ME, Biscotti, CV, Triozzi, PL (2016). Fine-needle aspiration biopsy of uveal melanoma: outcomes and complications. Br J Ophthalmol. 100. https://doi.org/10.1136/bjophthalmol-2015-306921.
Sen J, Groenewald C, Hiscott PS, Smith PA, Damato BE. Transretinal choroidal tumor biopsy with a 25-gauge vitrector. Ophthalmology. 2006;113. https://doi.org/10.1016/j.ophtha.2006.02.048.
Bechrakis NE, Foerster MH, Bornfeld N. Biopsy in indeterminate intraocular tumors. Ophthalmology. 2002;109. https://doi.org/10.1016/S0161-6420(01)00931-9.
Chang MY, McCannel TA. Comparison of uveal melanoma cytopathologic sample retrieval in Trans-scleral versus vitrectomyassisted transvitreal fine needle aspiration biopsy. Br J Ophthalmol. 2014;98. https://doi.org/10.1136/bjophthalmol-2014-305181.
Frizziero L, Midena E, Trainiti S, Londei D, Bonaldi L, Bini S, et al. Uveal melanoma biopsy: a review. Cancers. 2019;11. https://doi.org/10.3390/cancers11081075.
Hussain R, Heussen FM, Heimann H. OCT changes in peri-tumour normal retina following ruthenium-106 and proton beam radiotherapy for uveal melanoma. Br Jof Ophthalmol. 2021;105. https://doi.org/10.1136/bjophthalmol-2019-314802.
Fujiwara N, Tomita G, Yagi F. Incidence and risk factors of iatrogenic retinal breaks: 20-gauge versus 25-gauge vitrectomy for idiopathic macular hole repair. J Ophthalmol. 2020. https://doi.org/10.1155/2020/5085180.
Ramkissoon YD, Aslam SA, Shah SP, Wong SC, Sullivan PM. Risk of iatrogenic peripheral retinal breaks in 20-G pars plana vitrectomy. Ophthalmology. 2010;117. https://doi.org/10.1016/j.ophtha.2010.01.029.
Grewal DS, Bhullar PK, Pasricha ND, Carrasco-Zevallos OM, Viehland C, Keller B, et al. Intraoperative 4-dimensional microscope-integrated optical coherence tomography-guided 27-gauge transvitreal choroidal biopsy for choroidal melanoma. Retina. 2017;37:796–9. https://doi.org/10.1097/IAE.0000000000001326. PMID: 27673716; PMCID: PMC5362285.
Murray TG, Gold AS, Markoe AM, Wildner A, Latiff A, Bermudez E, et al. Spectral-domain optical coherence tomography evaluation of choroidal melanoma and nevus fine-needle aspiration biopsy incision sites. Ophthalmic Surg, Lasers Imaging Retin. 2014;45:165–8. https://doi.org/10.3928/23258160-20140306-11.
Hussain RN, Kalirai H, Groenewald C, Kacperek A, Errington RD, Coupland SE, et al. Prognostic biopsy of choroidal melanoma after proton beam radiation therapy. Ophthalmology. 2016;123. https://doi.org/10.1016/j.ophtha.2016.05.028.
Author information
Authors and Affiliations
Contributions
HS was responsible for conducting the image assessment after identifying eligible patients, data collection and analyses, interpretation of results, updating reference lists and writing of the manuscript. AT aided in statistical analysis of data. LG reviewed the manuscript and assisted in writing the manuscript. HH contributed to the study design, interpretation of results, updating reference lists, feedback and editing the manuscript. RH contributed to the study design, assessment of image, updating reference lists and interpretation of results feedback and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haider, S., Azam, T., Leandro, G. et al. Evaluation of optical coherence tomography (OCT) changes following transretinal tumour biopsy for choroidal melanoma. Eye 37, 1014–1018 (2023). https://doi.org/10.1038/s41433-022-02185-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02185-1
This article is cited by
-
Ocular oncology demystified
Eye (2022)