Learning Objectives
Upon completion of this activity, participants will be able to:
-
1.
Describe the overall relationship between retinal vascular parameters and foveal avascular zone (FAZ) architecture and age in normal healthy eyes over a wide age range, according to a cross-sectional study using swept-source optical coherence tomography angiography (SS OCTA).
-
2.
Determine the relationship between retinal vascular parameters and FAZ architecture and age in normal healthy eyes according to macular region and specific decades of life, according to a cross-sectional study using SS OCTA.
-
3.
Identify clinical implications of the relationship between retinal vascular parameters and FAZ architecture and age in normal healthy eyes over a wide age range, according to a cross-sectional study using SS OCTA.
Accreditation Statements
In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and Springer Nature. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at www.medscape.org/journal/eye; (4) view/print certificate.
Credit hours
1.0

Release date:
Expiration date:
Post-test link: https://medscape.org/eye/posttest972903
Journal CME author disclosure information
Laurie Barclay has disclosed the following relevant financial relationships: Owns stock, stock options, or bonds from: AbbVie Inc. (former).
Abstract
Aim
To assess the macular capillary networks and foveal avascular zone (FAZ) with swept-source optical coherence tomography angiography in healthy eyes.
Methods
This cross-sectional, prospective, observational study enrolled 222 eyes of 116 healthy participants with no ocular or systemic disease. SS-OCTA images were captured using the PLEX Elite 9000 (Carl Zeiss Meditec Inc., Dublin, CA, USA) with a 6 × 6 mm pattern centered on the foveal center. Vessel length density (VLD), perfusion density (PD), and FAZ parameters were analyzed using the manufacturer’s automated software.
Results
A significant negative correlation was observed between age and average VLD in the superficial plexus, and average PD in both the superficial plexus and the whole retina. A significant positive correlation between age and foveal avascular zone perimeter and area was also noted. Age-wise comparisons showed a trend for an increase in VLD and PD until 40 years of age, with a subsequent decrease in the older age in the macular region. The central subfield showed a decrease in the vessel density measurements in the 21–40 age group. FAZ area and perimeter were the mirror inverse of the central subfield vessel density measurements with a numerically greater area and perimeter in the 21–40 age group compared to the 0–20 and 41–60 age groups. FAZ circularity was significantly reduced after 40 years of age.
Conclusion
Age-related changes in the vessel density and FAZ parameters in the healthy macula are complex and vary with the macular location. These results carry significance when interpreting the data from diseased eyes.
Similar content being viewed by others
Introduction
Optical coherence tomography angiography (OCTA) has significantly enhanced our knowledge of the retinal and inner choroidal microcirculation. OCTA, which utilizes decorrelation signals from OCT scans obtained over time, allows the circulation to be visualized in a depth-resolved volumetric fashion [1, 2], offering significant advantages over conventional dye-based fundus fluorescein angiography and indocyanine green angiography [3]. While OCTA cannot currently demonstrate leakage which can provide insight into the integrity of the blood-retinal barrier, the absence of obscuring leakage provides a clearer visualization of the circulation, particularly the deeper layers. The deeper capillary layers can be specifically impacted in various retinal vascular diseases including diabetic retinopathy and retinal vascular occlusive disease [4,5,6,7,8]. A specific phenotype of selective occlusion of the deeper circulation, termed paracentral acute middle maculopathy (PAMM), has also been described [9].
Recently, OCTA has become available on swept-source (SS) OCT devices, which feature a tunable laser with a longer deeper-penetrating wavelength (1050 nm) and faster scanning speed. These devices offer some advantages over spectral-domain (SD) OCT devices for evaluation of the choriocapillaris and inner choroid [10].
OCTA has provided novel insights into the organization and physiology of the normal healthy vasculature. These include a detailed description of the number of layers in which the vascular plexuses are arranged, regional variations between the macular and peripapillary region, a hybrid model of series and parallel organization of the microvascular blood flow, with an apparent directional flow between the superficial (SCP) and deep capillary plexuses (DCP) and preferential venous outflow at the level of the DCP [11,12,13,14].
Since the macula is amongst the metabolically most active tissues of the body, it is affected by hypoxic conditions early on in many disease states [15,16,17]. It is supplied by multiple surrounding capillary plexuses for its high oxygen demand, and therefore, depends upon the health of these capillary beds for its proper integrity and function [18]. Understanding the normal circulation is a critical prerequisite for understanding the impact of diseases. Characterizing the variation in the normal circulation related to age, ethnicity, and retinal region is essential. Not surprisingly, as with other vascular systems, changes have been reported to occur in the retinal microvasculature with age [19, 20]. The alterations occurring in the foveal avascular zone (FAZ) and surrounding vessels with age in healthy eyes have been described in the past [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. Though the relationships are not perfectly consistent, generally, a decreased perifoveal vessel density and increased FAZ size have been reported with advancing age [23,24,25,26,27,28,29,30,31,32]. The perimeter of the FAZ is supplied by both the SCP and DCP which form a ring of interconnecting capillaries at the margin of the FAZ [36, 37]. The FAZ zone is known to be sensitive to ischemic events, and the shape and size of the normal FAZ correlate well with foveal morphology [33]. Recently, researchers have reported early evidence of alterations in the FAZ circularity in patients with diabetes as compared with healthy eyes [38,39,40]. One important reason for the lack of consistent results in all such studies may be related to the age of the study cohort included for analysis. Thus, precise information regarding age-related alterations in the FAZ and surrounding retinal vasculature becomes a prerequisite to understand the development in health and alterations in diseased states [41]. To address this need, we assessed normal healthy eyes over a wide age range using SS OCTA device which permits dense scanning of the macular circulation to unravel the complex variations in these structures as the age advances.
Methods
Study design
This was a cross-sectional, observational study conducted at the vitreoretinal clinic, Sankara Nethralaya, Chennai, India with subjects recruited among patients reporting for a routine eye evaluation between January 2019 through July 2020. It was approved by the Institutional Review Board of the Vision Research Foundation, Chennai. The study was performed in accordance with the Health Insurance Portability and Accountability Act, and adhered to the tenets of the Declaration of Helsinki. All the participants/guardians signed a written consent prior to enrollment.
Study participants
In this study, we evaluated 222 eyes of 116 healthy participants with no ocular or systemic disease. Major exclusion criteria included refractive error > +2 or < −3 diopter (D) spherical equivalent, glaucoma, intraocular inflammation, any previous history of intraocular surgery, laser or injections, presence of any media opacities. The presence of systemic disease was excluded based on detailed history from the patient and review of medical records and any medication use. None of the female participants were pregnant at the time of the study.
The participants’ demographic data, best-corrected visual acuity, and detailed ophthalmic examination findings including intraocular pressure by applanation tonometry were recorded. Slit-lamp examination and dilated fundus evaluation with indirect ophthalmoscopy were performed to exclude any ocular disease. Being a normative study, we chose to include both eyes of the healthy participants for final analysis, unless excluded as specified later.
Image acquisition and scanning protocols
SS-OCTA images were captured using the PLEX Elite 9000 (Carl Zeiss Meditec Inc., Dublin, CA, USA), which uses a swept laser source with a central wavelength of 1050 nm (100,000 A-scans per second) and an axial resolution of about 2 µ in tissue, operating at a bandwidth of 100 nm, an A-scan depth of 3.0 mm in tissue, an axial optical resolution of about 6.3 μm and a transverse resolution of approximately 20 μm. For this study, 6 × 6 mm OCTA scans centered on the foveal center were acquired for each eye. Each volume scan consisted of dense pattern of 500 B-scans × 500 A-scans repeated at least two times and captured with the instruments FastTrac eye motion artifact correction software (Carl Zeiss Meditec, Inc, USA). The device uses the optical microangiography (OMAG) to identify motion and visualize the capillary networks with high resolution and contrast. The instrument’s automated retinal layer segmentation algorithm was utilized to define the retinal vascular layers. Automated analysis was accomplished using the cloud-based Advanced Retinal Imaging (ARI) Network hub made available by the manufacturer [42]. Images with poor image quality (defined by the presence of motion artifacts or signal strength index less than 7) or segmentation error were excluded.
Grading protocol
The following definitions/boundary positions were used for slab segmentation of the different layers, as established by the instrument manufacturer [43]. The whole retinal slab was defined by an inner segmentation line following the contour of the internal limiting membrane (ILM), and an outer segmentation line located 70 microns above the RPE-fit but following the contour of the RPE-fit. The superficial capillary plexus (SCP) was defined by a slab with the inner surface at the ILM and the outer surface at the inner two-thirds of the inner plexiform layer. The deep capillary plexus (DCP) extended from the outer border of the SCP slab to a surface just below the outer plexiform layer-outer nuclear layer interface.
Computation of vascular parameters
As previously noted, the automated measurements of all study vascular parameters were obtained through the manufacturer’s processing available through the ARI portal.
Vessel length density (VLD)
VLD (expressed as mm−1) was computed from the binarized and skeletonized image and defined as the fraction of the total length of vessel pixels divided by the total number of pixels in the region of interest. VLD was computed for the whole retina as well as for the SCP and the DCP individually.
Perfusion density
PD (unitless number) was computed from the binarized image as the total number of pixels with flow divided by the total number of pixels within the region of interest. PD was computed for the retina as a whole, as well as for the SCP and the DCP.
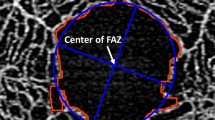
Foveal avascular zone (FAZ) measurements
Quantitative parameters to describe the FAZ morphology included perimeter (in mm), circularity, and area (in mm2). All FAZ measurements were based on the FAZ appearance in the SCP slab.
Data analysis and statistics
Statistical analysis was performed using SPSS (Statistical Package for Social Sciences, version 20.0, Chicago, IL, USA). A Shapiro–Wilk test was used to assess the normality assumptions. All tests used were two-sided with a type 1 error of 5%. One-way ANOVA was used to analyze the variation in retinal VLD (superficial, deep, and whole retina), PD (superficial, deep, and whole retina), and foveal avascular zone (circularity, perimeter, and area) between different age categories (group 1 to 4). Inter eye correlations were adjusted using generalized estimating equations during mean difference analysis. Bonferroni post hoc test was used to compare the mean values between the subgroups by considering a conservative p-value. Pearson correlation was performed between the study parameters and age. Linear regression analysis was performed to assess age-related changes in the study parameters. Inter-grader agreement for all the quantitative parameters was assessed using Cohen’s kappa coefficient (κ).
Results
We studied 222 eyes from 116 healthy patients, of which 72 (62%) subjects were females. The refractive errors ranged from +2.0 D to −3.0 D. The mean intraocular pressure by applanation tonometry was 12 mm Hg (range 10–16 mm Hg). Table 1 shows the average values of the study parameters as observed in every decade of life. Notably, the difference in the parameters was not statistically significant between the groups when compared in each decade of life. Table 2 shows the correlation between age and various vascular parameters within the entire 6 × 6 scan area. A significant negative correlation was observed between age and average VLD (P = 0.01) in the superficial plexus, and average PD in both the superficial plexus (P = 0.001) and whole retina (P = 0.001). In addition, we observed a significant positive correlation between age and FAZ perimeter and area.
We also analyzed the correlation between age and the vessel parameters (VLD and PD) within the rings of the ETDRS grid (Table 3). Of note, both the VLD and PD showed a significant negative correlation with age in both the 1 and 3 mm ETDRS rings. However, in the 6 mm ETDRS ring, this correlation persisted only for the VLD in SCP, and the PD in both the SCP and whole retina.
Scatter plots in Fig. 1 illustrate the relationship between the study parameters and age. The vascular parameters (Fig. 1A–C, VLD; Fig. 1D–F, PD) showed a downward deflection of the best-fit line after the 3rd and 4th decades. When comparing plots, the correlation appears to be superior for VLD and PD from the SCP and whole retina, and the FAZ perimeter. Similarly, a positive correlation was noted between age and the FAZ perimeter and area (Fig. 1G and I, respectively), with clustering of the data points close to the best-fit line. The bar graphs in Fig. 2A–D (Fig. 2A: ETDRS 1 mm central subfield; Fig. 2B: ETDRS 3 mm ring; Fig. 2C: ETDRS 6 mm ring; Fig. 2D: whole ETDRS region) and Fig. 2E–G (FAZ area, perimeter, and circularity) illustrate the changes in study parameters for every two decades of life. For this analysis, the study cohort was divided (pre-specified) age-wise as follows: group 1 (<20 years; N = 34; eyes = 65); group 2 (21–40 years; N = 41; eyes = 81); group 3 (41–60 years; N = 32; eyes = 62); and group 4 (>61 years; N = 9; eyes = 14). In addition to the ANOVA across all groups, p-values for pairwise comparisons between the subgroups were performed. Overall, there was a trend for an increase in VLD and PD between the 0–20 and 21–40 age groups with a subsequent decrease in the older groups in the more peripheral rings (Fig. 2B and C). In the central subfield (1 mm ring), however, the VLD and PD were numerically lower in the 21–40 age group compared to the 0–20 and 41–60 age groups (Fig. 2A). For all VLD and PD measurements in all subfields, the values were lowest in the 61–80 years subgroup. When the whole 6 mm ETDRS circle was analyzed, it followed the trend as in 3 mm and 6 mm rings i.e., the VLD and PD increased till about 40 years, with a decline thereafter, with least values in 61–80 years age group (Fig. 2D).
FAZ perimeter and area (Fig. 2E–G) were the mirror inverse of the central subfield VLD and PD measurements with a numerically greater area and perimeter in the 21–40 age group compared to the 0–20 and 41–60 age groups. The circularity of the FAZ was significantly lower in the 41–60 and 61–80 age groups compared to the younger age groups.
We found no significant differences between males and females in this study.
A high level of consistency between two graders (AV and KM) with an unweighted κ values of 0.95 (95% confidence interval, CI 0.91–0.99) for VLD, 0.96 (95% CI 0.91–0.99) for PD, and 0.92 (95% CI 0.88–0.96) for FAZ parameters were obtained.
Discussion
In this study, we evaluated the relationship between retinal vascular parameters and FAZ architecture and age using swept-source OCT angiography. Overall, our results suggest that there is a decrease in vessel and perfusion density in the macula and increase in FAZ area and perimeter with age. However, a more granular analysis according to macular region and specific decades of life suggest a more complex relationship. Within the ETDRS rings, when analyzed by every two decades of life, the vessel density parameters actually showed an increasing trend till about 40 years of age, with a gradual decline thereafter. However, in the central subfield (1 mm ring), the inverse was observed with a reduction in these parameters at 21–40 years of age. However, the lowest vessel density values were seen in the older age group, beyond 61 years. The FAZ parameters showed an increasing trend until 40 years of age, with a decline by the 41–60 years age group. The largest values for FAZ area and perimeter were noted beyond 60 years of age, whereas the circularity did not change. Thus, the FAZ measurement was essentially the inverse of the vessel density parameters in the central subfield. This makes sense since assuming similar capillary spacing, a larger FAZ will leave less area for surrounding vessels in the foveal central subfield. The scatter plots (Fig. 1A–F) suggested that overall, there is an initial trend for increasing vessel density parameters which ultimately drop to a value lower than the baseline by old age. Analogously, an eventual increase in FAZ area and perimeter, and a decrease in circularity with older age were also evident (Fig. 1G–I).
Our study expands upon previous studies of OCTA-derived retinal vessel parameters in normal eyes. Previous studies investigating the relationship with age have demonstrated inconsistent results with a non-uniform trend [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35, 44]. This may be in part due to geographical and ethnic variations as well as different scanning platforms and imaging algorithms that were used to generate the results. Iafe et al. in their study reported a decrease in VD and an increase in FAZ area with age [23]. Falavarjani et al. showed that the FAZ area in the DCP was negatively correlated with age, but that of the SCP was not [41].
Our study establishes for the first time, however, that the relationship with age may be more complicated, with differences based on the macular region and changes going in one direction until age 40 and then moving in the opposite direction after that. Not surprisingly, the FAZ-related parameters (area and perimeter in particular) appeared to be most closely linked to the foveal central subfield (1 mm ring) vessel density and perfusion density parameters, showing an analogous, but opposite relationship with age.
Previous studies assessing the relationship between age and FAZ size have also yielded inconsistent results [23, 41, 45,46,47,48,49,50,51]. We observed a slight increase in the 3rd and 4th decade (FAZ area, circularity and perimeter), and again at the 7th decade onwards (FAZ area and perimeter), with the highest values noted at or beyond 7th decades. Various factors apart from age, such as hormonal changes, geographical/ethnic variations, and the imaging technique have been found to influence the FAZ architecture [52, 53]. Hsu et al. evaluated the macular region of infants and children using OCTA and reported a significant variation related to the age, race, and axial length [34]. They reported a slight increase in PD and VLD at about 10–15 years of age, which starts to decline thereafter. Our results showed that the VLD, PD, and the FAZ area and perimeter show a gradual increasing trend till about 40 years of age. These changes may coincide with the final attainment of foveal maturity which occurs at about the same age [54]. Subsequent changes after age 40 (once complete maturity has been achieved) may reflect the degenerative impact of aging. Therefore, this study analyzing the effect of age is an extension of the existing literature supporting the association of FAZ architecture with vessel indices in healthy eyes, which may be of relevance when analyzing diseased eyes. Interestingly, the fact that the vessel density parameters were highest in the central subfield in 0–20 years age group reflects an important aspect of development of the human fovea. As the investigations have revealed that FAZ develops with the development of foveal pit, the vessel density is bound to be highest in early years of age, with a subsequent gradual decline [55,56,57]. The measurements of the central subfield vessel density parameters and FAZ parameters showed and inverse relationship.
FAZ circularity showed a slight increase at the 3rd and 4th decades, with a significant decline over the next two decades, with no gross changes thereafter. Various studies have compared FAZ circularity between healthy and diabetic eyes. The eyes with ischemic alterations showed altered and decreased circularity [58,59,60,61], however, healthy eyes and diabetic eyes without diabetic retinopathy had comparable circularity indices [38]. Also, Shiihara et al. reported a lack of association between age with FAZ shape in their study, but they did not divide their cohort into specific age groups as in our analysis [62]. The increase in FAZ circularity in the 21–40 age group in our study may reflect the progressive maturation of the macula in the initial decades of life. However, larger longitudinal studies will be necessary to confirm our observations.
Our study has a number of limitations which must be considered when assessing our results. First, we relied on the slab definitions for the SCP and DCP and the manufacturer’s automated segmentation for the various vessel indices. While these algorithms work very well in normal eyes and no gross errors were seen on manual inspection, we cannot exclude subtle errors. On the other hand, use of automated results may make our findings more broadly applicable as they would be available to all clinicians and researchers using these devices. Second, the number of subjects in our oldest subgroup was small and notably smaller than the others which reduces the overall power of the study. A larger confirmatory study with more subjects will be important to expose additional results, which may differ from this study. Third, our study was cross-sectional and we can only infer changes that may be occurring to retinal vessel parameters with age. A long-term longitudinal study would be the best to definitively assess this. As the study cohort comprised of Indian population, the results cannot be generalized to different ethnicities and geographical locales. We do not have repeat examinations of the same subject (being a cross-sectional study) to establish inter-visit repeatability though that has been address in prior papers [63]. Lei et al. have reported a high level of repeatability and reproducibility when measuring retinal microvasculature using OCTA. Finally, our data is limited to a single OCTA instrument, and thus we cannot definitively establish that similar findings would be observed with other devices. Our study also has many strengths including a prospective design, a wide distribution of ages, and the use of strict exclusion criteria to limit any variations due to ocular (e.g., axial length) or systemic parameters.
In summary, we demonstrate that there are age-related differences in vessel parameters on OCTA in normal eyes. These differences appear to depend on macular region as well as the specific decade of life. Understanding these normal age-related differences may provide new insights into normal retinal development and aging and will be essential to interpret the impact of retinal diseases.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50.
Moult E, Choi W, Waheed NK, Adhi M, Lee B, Lu CD, et al. Ultrahigh-speed swept-source OCT angiography in exudative AMD. Ophthalmic Surg Lasers Imaging Retin. 2014;45:496–505.
Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20:4710–25.
Wu LZ, Huang ZS, Wu DZ, Chan E. Characteristics of the capillary-free zone in the normal human macula. Jpn J Ophthalmol. 1985;29:406–11.
Eldaly Z, Soliman W, Sharaf M, Reyad AN. Morphological characteristics of normal foveal avascular zone by optical coherence tomography angiography. J Ophthalmol. 2020;2020:8281459.
Carnevali A, Sacconi R, Corbelli E, Tomasso L, Querques L, Zerbini G, et al. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017;54:695–702.
Freiberg FJ, Pfau M, Wons J, Wirth MA, Becker MD, Michels S. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:1051–8.
Tsai G, Banaee T, Conti FF, Singh RP. Optical coherence tomography angiography in eyes with retinal vein occlusion. J Ophthalmic Vis Res. 2018;13:315–32.
Chen X, Rahimy E, Sergott RC, Nunes RP, Souza EC, Choudhry N, et al. Spectrum of retinal vascular diseases associated with paracentral acute middle maculopathy. Am J Ophthalmol. 2015;160:26–.e1.
Gorczynska I, Migacz JV, Jonnal R, Zawadzki RJ, Poddar R, Werner JS. Imaging of the human choroid with a 1.7 MHz A-scan rate FDML swept source OCT system. In Progress in Biomedical Optics and Imaging - Proceedings of SPIE, 10045, art. no. 1004510 (2017).
Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 2017;7:42201.
Scharf J, Freund KB, Sadda S, Sarraf D. Paracentral acute middle maculopathy and the organization of the retinal capillary plexuses. Prog Retin Eye Res. 2021;81:100884.
Garrity ST, Paques M, Gaudric A, Freund KB, Sarraf D. Considerations in the understanding of venous outflow in the retinal capillary plexus. Retina. 2017;37:1809–12.
Freund KB, Sarraf D, Leong BCS, Garrity ST, Vupparaboina KK, Dansingani KK. Association of optical coherence tomography angiography of collaterals in retinal vein occlusion with major venous outflow through the deep vascular complex. JAMA Ophthalmol. 2018;136:1262–70.
Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20:175–208.
Yu DY, Cringle SJ, Su EN. Intraretinal oxygen distribution in the monkey retina and the response to systemic hyperoxia. Invest Ophthalmol Vis Sci. 2005;46:4728–33.
Yu DY, Yu PK, Cringle SJ, Kang MH, Su EN. Functional and morphological characteristics of the retinal and choroidal vasculature. Prog Retin Eye Res. 2014;40:53–93.
Hassan M, Sadiq MA, Halim MS, Afridi R, Soliman MK, Sarwar S, et al. Evaluation of macular and peripapillary vessel flow density in eyes with no known pathology using optical coherence tomography angiography. Int J Retin Vitreous. 2017;3:27.
Grunwald JE, Piltz J, Patel N, Bose S, Riva CE. Effect of aging on retinal macular microcirculation: a blue field simulation study. Invest Ophthalmol Vis Sci. 1993;34:3609–13.
Laatikainen L, Larinkari J. Capillary-free area of the fovea with advancing age. Invest Ophthalmol Vis Sci. 1977;16:1154–7.
Gadde SG, Anegondi N, Bhanushali D, Chidambara L, Yadav NK, Khurana A, et al. Quantification of vessel density in retinal optical coherence tomography ngiography images using local fractal dimension. Invest Ophthalmol Vis Sci. 2016;57:246–52.
Yu J, Gu R, Zong Y, Xu H, Wang X, Sun X, et al. Relationship between retinal perfusion and retinal thickness in healthy subjects: an optical coherence tomography angiography study. Invest Ophthalmol Vis Sci. 2016;57:204–10.
Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Retinal capillary density and foveal avascular zone area are age-dependent: quantitative analysis using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:5780–7.
Leng Y, Tam EK, Falavarjani KG, Tsui I. Effect of age and myopia on retinal microvasculature. Ophthalmic Surg Lasers Imaging Retin. 2018;49:925–31.
Shahlaee A, Samara WA, Hsu J, Say EA, Khan MA, Sridhar J, et al. In vivo assessment of macular vascular density in healthy human eyes using optical coherence tomography angiography. Am J Ophthalmol. 2016;165:39–46.
Pujari A, Chawla R, Markan A, Shah P, Kumar S, Hasan N, et al. Age‑related changes in macular vessels and their perfusion densities on optical coherence tomography angiography. Indian J Ophthalmol. 2020;68:494–9.
Wei Y, Jiang H, Shi Y, Qu D, Gregori G, Zheng F, et al. Age-related alterations in the retinal microvasculature, microcirculation, and microstructure. Invest Ophthalmol Vis Sci. 2017;58:3804–17.
Coscas F, Sellam A, Glacet-Bernard A, Jung C, Goudot M, Miere A, et al. Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:211–23.
Yu J, Jiang C, Wang X, Zhu L, Gu R, Xu H, et al. Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Opthalmol Vis Sci. 2015;56:3212–7.
Pinhas A, Linderman R, Mo S, Krawitz BD, Geyman LS, Carroll J, et al. A method for age-matched OCT angiography deviation mapping in the assessment of disease-related changes to the radial peripapillary capillaries. PLoS ONE. 2018;13:e0197062.
Ghassemi F, Mirshahi R, Bazvand F, Fadakar K, Faghihi H, Sabour S. The quantitative measurements of foveal avascular zone using optical coherence tomography angiography in normal volunteers. J Curr Ophthalmol. 2017;29:293–9.
Lavia C, Bonnin S, Maule M, Erginay A, Tadayoni R, Gaudric A. Vessel density of superficial, intermediate, and deep capillary plexuses using optical coherence tomography angiography. Retina. 2019;39:247–58.
Samara WA, Say EA, Khoo CT, Higgins TP, Magrath G, Ferenczy S, et al. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina. 2015;35:2188–95.
Hsu ST, Ngo HT, Stinnett SS, Cheung NL, House RJ, Kelly MP, et al. Assessment of macular microvasculature in healthy eyes of infants and children using OCT angiography. Ophthalmology. 2019;126:1703–11.
Sato R, Kunikata H, Asano T, Aizawa N, Kiyota N, Shiga Y, et al. Quantitative analysis of the macula with optical coherence tomography angiography in normal Japanese subjects: the Taiwa Study. Sci Rep. 2019;9:8875.
Snodderly DM, Weinhaus RS, Choi JC. Neural-vascular relationships in central retina of macaque monkeys (Macaca fascicularis). J Neurosci. 1992;12:1169–93.
Paques M, Tadayoni R, Sercombe R, Laurent P, Genevois O, Gaudric A, et al. Structural and hemodynamic analysis of the mouse retinal microcirculation. Invest Ophthalmol Vis Sci. 2003;44:4960–7.
Somilleda-Ventura SA, Razo-Blanco-Hernández DM, Reyes-Calderón JA, Ceballos-Reyes GM, Lima-Gómez V. Circularity of the foveal avascular zone and its correlation with parafoveal vessel density, in subjects with and without diabetes. Cir Cir. 2019;87:390–5.
Krawitz BD, Mo S, Geyman LS, Agemy SA, Scripsema NK, Garcia PM, et al. Acircularity index and axis ratio of the foveal avascular zone in diabetic eyes and healthy controls measured by optical coherence tomography angiography. Vis Res. 2017;139:177–86.
Tang FY, Ng DS, Lam A, Luk F, Wong R, Chan C, et al. Determinants of quantitative optical coherence tomography angiography metrics in patients with diabetes. Sci Rep. 2017;7:2575.
Falavarjani KG, Shenazandi H, Naseri D, Anvari P, Kazemi P, Aghamohammadi F, et al. Foveal avascular zone and vessel density in healthy subjects: an optical coherence tomography angiography study. J Ophthalmic Vis Res. 2018;13:260–5.
Tumlinson AR, Rahman F, Magazzeni S, Pochendorfer P, Shemonski N. The advanced retina imaging network hub online tools for OCT innovation. Invest Ophthalmol Vis Sci 2017;58:1864.
Lavia C, Mecê P, Nassisi M, Bonnin S, Marie-Louise J, Couturier A, et al. Retinal capillary plexus pattern and density from fovea to periphery measured in healthy eyes with swept-source optical coherence tomography angiography. Sci Rep. 2020;10:1474.
Shahlaee A, Pefkianaki M, Hsu J, Ho AC. Measurement of foveal avascular zone dimensions and its reliability in healthy eyes using optical coherence tomography angiography. Am J Ophthalmol. 2016;161:50–5.e1.
Fujiwara A, Morizane Y, Hosokawa M, Kimura S, Shiode Y, Hirano M, et al. Factors affecting foveal avascular zone in healthy eyes: an examination using swept-source optical coherence tomography angiography. PLoS ONE. 2017;12:e0188572.
Gariano RF, Provis JM, Hendrickson AE. Development of the foveal avascular zone. Ophthalmology. 2000;107:1026.
Provis JM, Hendrickson AE. The foveal avascular region of developing human retina. Arch Ophthalmol. 2008;126:507–11.
Mintz-Hittner HA, Knight-Nanan DM, Satriano DR, Kretzer FL. A small foveal avascular zone may be an historic mark of prematurity. Ophthalmology. 1999;106:1409–13.
Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55.
Bresnick GH, Condit R, Syrjala S, Palta M, Groo A, Korth K. Abnormalities of the foveal avascular zone in diabetic retinopathy. Arch Ophthalmol. 1984;102:1286–93.
de Carlo TE, Chin AT, Bonini Filho MA, Adhi M, Branchini L, Salz DA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35:2364–70.
Hendrickson A, Possin D, Vajzovic L, Toth CA. Histologic development of the human fovea from mid gestation to maturity. Am J Ophthalmol. 2012;154:767–78.e2.
Ogueta SB, Schwartz SD, Yamashita CK, Farber DB. Estrogen receptor in the human eye: influence of gender and age on gene expression. Invest Ophthalmol Vis Sci. 1999;40:1906–11.
Yu MC, Li WW, Liu K, Yew DT. An immunohistochemical study of the c-fos protooncogene in the developing human retina. Neuroscience. 1994;60:983–7.
Henkind P, Bellhorn RW, Murphy ME, Roa N. Development of macular vessels in monkey and cat. Br J Ophthalmol. 1975;59:703–9.
Gariano RF, Sage EH, Kaplan HJ, Hendrickson AE. Development of astrocytes and their relation to blood vessels in fetal monkey retina. Invest Ophthalmol Vis Sci. 1996;37:2367–75.
Hendrickson A, Troilo D, Possin D, Springer A. Development of the neural retina and its vasculature in the marmoset Callithrix jacchus. J Comp Neurol. 2006;497:270–86.
Freiberg FJ, Pfau M, Wons J, Wirth MA, Becker MD, Michels S. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:1051–8.
Cicinelli MV, Carnevali A, Rabiolo A, Querques L, Zucchiatti I, Scorcia V, et al. Clinical spectrum of macular-foveal capillaries evaluated with optical coherence tomography angiography. Retina. 2017;37:436–43.
Conrath J, Giorgi R, Raccah D, Ridings B. Foveal avascular zone in diabetic retinopathy: quantitative vs qualitative assessment. Eye. 2005;19:322–6.
Samara WA, Say EA, Khoo CT, Higgins TP, Magrath G, Ferenczy S, et al. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina. 2015;35:2188–95.
Shiihara H, Terasaki H, Sonoda S, Kakiuchi N, Shinohara Y, Tomita M, et al. Objective evaluation of size and shape of superficial foveal avascular zone in normal subjects by optical coherence tomography angiography. Sci Rep. 2018;8:10143.
Lei J, Durbin MK, Shi Y, Uji A, Balasubramanian S, Baghdasaryan E, et al. Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmol. 2017;135:1092–8.
Acknowledgements
We acknowledge the collaboration of the Department of Vitreoretina (Shri Bhagwan Mahavir Vitreoretinal Services) and the Department of Optometry in collecting and analyzing the data.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly for data collection, analysis and drafting of the manuscript. KM and AT significantly helped in recruiting the patients, AV and SRS contributed significantly for the study design, and drafting, and formatting the manuscript.
Corresponding author
Ethics declarations
Competing interests
SRS has the following financial disclosures: Consultant: Amgen, Allergan, Genentech/Roche, Iveric, Oxurion, Novartis, Regeneron, Bayer, 4DMT, Centervue, Heidelberg, Optos, Merck, Apellis, Astellas; Speaker Fees: Carl Zeiss Meditec, Nidek; Research Instruments: Nidek, Topcon, Heidelberg, Carl Zeiss Meditec, Optos, Centervue. The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verma, A., Magesan, K., Amose, T. et al. Age-related assessment of foveal avascular zone and surrounding capillary networks with swept source optical coherence tomography angiography in healthy eyes. Eye 36, 1857–1864 (2022). https://doi.org/10.1038/s41433-022-02146-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02146-8
This article is cited by
-
Fractal analysis of the macular region in healthy eyes using swept-source optical coherence tomography angiography
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)