Abstract
Background/Objectives
To evaluate the ability of swept-source optical coherence tomography (SS-OCT) implemented with angiography analysis (SS-OCTA) to detect neuro-retinal and vasculature changes in patients with Parkinson’s disease (PD) and essential tremor (ET), and to distinguish between both pathologies.
Subjects/Methods
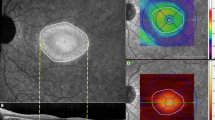
A total 42 PD and 26 ET patients and 146 controls underwent retinal evaluation using SS-OCT plus OCT-Angio™. The macular (m) and peripapillary (p) retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL), and macular vasculature were assessed. A Linear discriminant function (LDF) was calculated to evaluate the diagnostic ability of SS-OCTA in both PD and ET.
Results
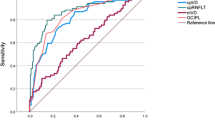
PD patients presented a reduction in mRNFL (p < 0.005), mGCL (all sectors, p < 0.05) and pRNFL (p < 0.005) vs healthy controls, and in mRNFL and pRNFL vs ET patients (p < 0.001). ET patients showed a significant reduction in mGCL vs controls (p < 0.001). No differences were observed in the macular vasculature between groups. Predictive diagnostic variables were significant only for PD and a LDF was obtained with an area under the ROC curve of 0.796.
Conclusions
Neuro-retinal thinning is present in both diseases, being greater in PD. While SS-OCT could be useful in diagnosing ET and PD, the diagnostic potential for SS-OCTA based on an LDF applies only to PD, not ET.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data supporting the conclusions of this study will be available upon reasonable request to the corresponding author.
References
Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–41.
Louis ED. The primary type of tremor in essential tremor is kinetic rather than postural: cross-sectional observation of tremor phenomenology in 369 cases. Eur J Neurol. 2013;20:725–7.
Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: Prevalence and association with disease duration. Mov Disord. 2009;24:626–7.
Rao AK, Louis ED. Ataxic Gait in Essential Tremor: A Disease-Associated Feature? Tremor Other Hyperkinet Mov (NY). 2019 9. https://doi.org/10.7916/d8-28jq-8t52
Benito-Leon J, Labiano-Fontcuberta A. Linking Essential Tremor to the Cerebellum: Clinical Evidence. Cerebellum 2016;15:253–62.
Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013;28:1759–61.
Marin-Lahoz J, Gironell A. Linking Essential Tremor to the Cerebellum: Neurochemical Evidence. Cerebellum 2016;15:243–52.
Khoo TK, Yarnall AJ, Duncan GW, Coleman S, O’Brien JT, Brooks DJ, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 2013;80:276–81.
Kwon DH, Kim JM, Oh SH, Jeong HJ, Park SY, Oh ES, et al. Seven-Tesla magnetic resonance images of the substantia nigra in Parkinson disease. Ann Neurol. 2012;71:267–77.
Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, et al. Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol. 2009;127:737–41.
Fekete R, Jankovic J. Revisiting the relationship between essential tremor and Parkinson’s disease. Mov Disord. 2011;26:391–8.
Minen MT, Louis ED. Emergence of Parkinson’s disease in essential tremor: a study of the clinical correlates in 53 patients. Mov Disord. 2012;23:1602–5.
Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson’s disease. Vis Res. 2004;44:2793–97.
Satue M, Obis J, Alarcia R, Rodrigo MJ, Vilades E. Gracia et al. Retinal and choroidal changes in patients with Parkinson’s disease detected by Swept source Optical coherence tomography. Curr Eye Res. 2018;43:109–15.
Tsokolas G, Tsaousis KT, Diakonis VF, Matsou A, Tyradellis S. Optical Coherence Tomography Angiography in Neurodegenerative Diseases: A Review. Eye Brain. 2020;12:73–87.
Cordon B, Vilades E, Orduna E, Satue M, Perez-Velilla J, Sebastian B, et al. Angiography with optical coherence tomography as a biomarker in multiple sclerosis. PLoS ONE. 2020;15:e0243236.
Tugcu B, Melikov A, Yildiz GB, Gökcal E, Ercan R, Uysal O, et al. Evaluation of retinal alterations in Parkinson disease and tremor diseases. Acta Neurol Belg. 2020;120:107–13.
Cubo E, Tedejo RP, Rodriguez-Mendez V, López-Peña MJ, Trejo-Gabriel Y, Galán JM. Retina thickness in Parkinson’s disease and essential tremor. Mov Disord. 2010;25:2461–2.
Turkel Y, Ornek N, Dag E, Ornek K, Alpua M, Ogurel T, et al. Retinal nerve fiber layer thickness in patients with essential tremor. Neurol Asia. 2015;20:363–6.
Reichmann H. Clinical criteria for the diagnosis of Parkinson’s disease. Neurodegenerative Dis. 2010;7:284–90. https://doi.org/10.1159/000314478
Satue M, Garcia-Martin E, Fuertes I, Otin S, Alarcia R, Dolz I, et al. Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson’s disease patients. EYE (Lond). 2013;27:507–14.
Polo V, Satue M, Rodrigo MJ, Otin S, Alarcia R, Bambo MP, et al. Visual dysfunction and its correlation with retinal changes in patients with Parkinson’s disease: an observational cross-sectional study. BMJ Open. 2016;6:e009658.
Bodis-Wollner I. Retinopathy in Parkinson disease. J Neural Transm. 2009;116:1493–501.
Tak AZA, Şengül Y, Karadağ AS. Evaluation of thickness of retinal nerve fiber layer, ganglion cell layer, and choroidal thickness in essential tremor: can eyes be a clue for neurodegeneration? Acta Neurol Belg. 2018;118:235–41.
Herrero R, Garcia-Martin E, Almarcegui C, Ara JR, Rodriguez-Mena R, Martin J, et al. Progressive degeneration of the retinal nerve fiber layer in patients with multiple sclerosis. Investig Ophthalmol Vis Sci. 2012;53:8344–9.
Garcia-Martin E, Pablo LE, Herrero R, Satue M, Polo V, Larrosa JM, et al. Diagnostic ability of a linear discriminant function for Spectral domain optical coherence tomography in multiple sclerosis patients. Ophthalmology 2012;119:1705–11.
Garcia-Martin E, Polo V, Bambo MP, Pinilla J, Larrosa JM, Satue M, et al. Reliability and validity of Cirrus and Spectralis optical coherence tomography for detecting retinal atrophy in Alzheimer’s disease. EYE (Lond). 2014;28:680–90.
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007;130:3297–307.
Kwapong WR, Ye H, Peng C, Zhuang X, Wang J, Shen M, et al. Retinal microvascular impairment in the early stages of Parkinson’s disease. Investig Ophthalmol Vis Sci. 2018;59:4115–22.
Garcia-Martin E, Satue M, Otin S, Fuertes I, Alarcia R, Larrosa JM, et al. Retina measurements for diagnosis of Parkinson disease. Retina 2014;34:971–80.
Funding
This research received no specific funding from any agency in the commercial or not-for-profit sectors. This paper was supported by PI17/01726, PI17/01946 and PI20/00437 (Carlos III Health Institute), and by MAT2017-83858-C2-2 and PID2020-113281RB-C22 MINECO/AEI/ERDF, EU. MS was supported by the Juan Rodes program (Carlos III Health Institute, CM17/00010).
Author information
Authors and Affiliations
Contributions
MS was responsible for the conception and design, interpretation of data, drafting of paper and final approval of the version to be published. LC, AP, EV, BC and JME carried out the acquisition, analysis and interpretation of data, critical revision of draft and the final approval of the version to be published. EMC and EGM were responsible for the conception and design, interpretation of data, critical revision of draft and final approval of the version to be published: All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors report no competing interests. The authors alone are responsible for the content and writing of the paper. The authors have no proprietary or commercial interest in any materials discussed in this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Satue, M., Castro, L., Vilades, E. et al. Ability of Swept-source OCT and OCT-angiography to detect neuroretinal and vasculature changes in patients with Parkinson disease and essential tremor. Eye 37, 1314–1319 (2023). https://doi.org/10.1038/s41433-022-02112-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02112-4