Abstract
Background/Objective
To characterize incidence rates and identify risk factors for admission and mortality in patients with endogenous endophthalmitis (EE) in the United States (US).
Subjects/Methods
Patients with EE were identified using the Nationwide Emergency Department (NEDS) Database from 2006 to 2017 in this cross-sectional study. Subjects were required to have diagnoses of both endophthalmitis and septicaemia using contemporary International Classification of Diseases diagnosis codes. Incidence rates, mortality rates and demographics were evaluated. Risk factors for admission and mortality were identified using weighted logistic regression analysis.
Results
A total of 6400 patients with EE were identified. Incidence increased from 0.10 (95% confidence interval [CI]: 0.07–0.12) per 100,000 in the US population in 2006 to 0.25 (95% CI: 0.21–0.30) in 2017 (p < 0.05). Most were female (55.4%), insured with Medicare (53.5%), were in the first income quartile earnings (29.3%) [bottom 25% income bracket], lived in the South (40.5%), and presented to metropolitan teaching hospitals (66.6%). Mortality increased from 8.6% (95% CI: 3.8–18.3%) in 2006 to 13.8% (95% CI: 9.7–19.2%) in 2017 (p = 0.94). Factors predicting admission included older age (odds ratio [OR] 32.59; [95% CI 2.95–359.78]) and intravenous drug use (OR 14.90 [95% CI: 1.67–133.16]). Factors associated with increased mortality included: human immunodeficiency virus infection/immune deficiencies (OR 2.58 [95% CI: 1.26–5.28]), heart failure (OR 2.12 [95% CI: 1.47–3.05]), and hepatic infections/cirrhosis (OR 1.89 [95% CI: 1.28–2.79]). Pneumonia and renal/urinary tract infections (UTI) were associated with both increased hospital admission [(pneumonia OR 9.64 (95% CI: 1.25–74.35, p = 0.030), renal/UTI OR 4.09 (95% CI: 1.77–9.48)] and mortality [(pneumonia OR 1.64 (95% CI: 1.17–2.29, p = 0.030), renal/UTI OR 1.87 (95% CI: 1.18–2.97)]. Patients with diabetes mellitus (DM) had decreased odds ratio for mortality (OR 0.49 [95% CI: 0.33–0.73]).
Conclusion
EE has increased in incidence throughout US. The two systemic factors that conferred both an increase in mortality and admission were pneumonia, and renal/UTI. Additional exploration of the potential protective association of DM with decreased mortality in this context is needed.
Similar content being viewed by others
Introduction
Endogenous endophthalmitis (EE) comprises approximately 2–8% of all cases of endophthalmitis [1]. The eye becomes a secondary site of inoculation with hematogenous spread of infection from elsewhere. Since it is relatively infrequent, there have been few large-scale studies evaluating EE. Furthermore, symptoms are non-specific, with blurred vision as the most common presenting ocular symptom [2]. The diagnosis is missed in a substantial number of cases, with reports citing numbers ranging from 16% to 63% [3, 4]. Meanwhile, EE can have a rapid, debilitating course resulting in profound visual impairment. Thus, universal routine screening paradigms have been introduced for patients with bloodstream infections as a means for early identification and treatment (e.g., Candida species), but without proven success [5, 6]. Clinical suspicion for EE in the appropriate context for patients with systemic infections needs to be high; awareness of demographics patterns, and admission and mortality trends may therefore help dictate clinical management and elucidate patient outcomes.
There have been several studies limited to single centres evaluating demographics and mortality trends in patients with EE [7,8,9,10,11]. However there are a paucity of other studies that have used national databases to study EE, and none have focused on admission or mortality data [12, 13]. The primary goal of our study is to characterize patients who present to the emergency department (ED) with EE and evaluate risk factors for admission and mortality by examining these characteristics using data from a national database of EDs in the United States (US). We also assessed demographics, incidence, and temporal trends.
Materials/subjects and methods
Data source
The Nationwide Emergency Department Sample (NEDS) is the largest all-payer ED database in the US, and was developed by the Healthcare Cost and Utilization Project to provide both regional and national estimates of ED care [14]. This database consolidates the State Inpatient Databases and State Emergency Department Databases including both patients seen in the ED and subsequently admitted to the hospital, as well as visits that do not result in admission. The database contains roughly 145 million ED visits from 990 hospitals across 36 states nationwide, approximating a 20% stratified sample of hospital-owned EDs. NEDS uses discharge data and elements derived from International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD, Tenth Revision, Clinical Modification (ICD-10-CM). This study was performed with the approval of the Johns Hopkins University School of Medicine Institutional Review Board and in accordance with the Declaration of Helsinki.
Study population
All cases with an ICD-9-CM and ICD-10-CM code corresponding to endophthalmitis were identified. Selection of cases also required septicaemia as a diagnosis for increasing rigor of capturing EE. These codes used for identification of EE are listed in Table 1.
Study measures
Secondary variables were also identified using ICD-9-CM and ICD-10-CM codes. Data related to patient, visit, institution, and outcomes were evaluated. Demographic variables consisted of age, gender, insurance status, and income quartile. Institution variables included region, metropolitan status, teaching designation, and trauma centre designation. Visit variables encompassed median cost adjusted for inflation and month. Outcome variables included admission status and mortality. The database only coded mortality if it occurred within the same visit as the presentation, including both death during ED stay or death occurring during the length of admission.
Data analysis
Nationwide estimates were determined using sample weights provided by NEDS. Weights were provided in the database using discharge level weights that are provided in the database to represent the relative proportion of the total US inpatient hospital population for each record, allowing for calculation of national estimates. Annual incidence of such visits was calculated using census data; the total number of EE cases during the study period was divided by the total US population in the same period. Standard descriptive statistical methods were used for assessing demographics, and characteristics related to visit and institution. A univariate and multivariate weighted logistic regression model was used to determine demographic and visit characteristics associated with both hospital admission and mortality. Variables in this analysis included age (grouped into ≤20, 21–40, 41–60, 61–80, ≥81), sex, household income, primary payer, patient location, hospital region, teaching status of hospital, and the following comorbidities: diabetes mellitus (DM), human immunodeficiency virus (HIV)/immune deficiencies, heart failure, pneumonia, endocarditis, intravenous drug use (IVDU), gastrointestinal infections, renal/urinary tract infections, and cirrhosis/hepatic infections. Weighting was used throughout to identify these variables to account for changes in sampling procedure during this time period. Statistical significance was set at p < 0.05.
Results
Demographics and baseline characteristics
In total, there were 29,400 patients with any endophthalmitis diagnosis code (Table 1) identified from 2006 to 2017. This group was further refined to 6400 patients who were identified as having EE using a diagnosis code consistent with septicaemia, of whom 6307 patients were admitted (98.5%) (Table 1). Demographic data are shown in Table 2. Most (55.4%) patients were female, the majority (53.5%) were insured with Medicare, and the median age was 62 years (interquartile range [IQR]: 49–75). The income quartile distribution was evenly distributed with most patients living in the Southern region (40.5%). Most patients presented to non-trauma centre (42.3%), metropolitan teaching hospitals (66.6%).
National trends in incidence, cost and mortality
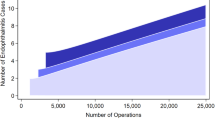
Incidence of EE increased from 0.10 (95% CI: 0.07–0.12) in 2006 to 0.25 (95% CI: 0.21–0.30) in 2017 (p < 0.05, Fig. 1). The median inflation-adjusted cost of ED visits for EE increased from $1229 (IQR: $690-$1563) in 2006 to $2529 (IQR: $1538–4038) in 2017 but was statistically insignificant (p = 0.23) (Fig. 2). There was no seasonal variation in the distribution of ED visits for EE (p = 0.46). Overall mortality rate was 12.9% and increased from 8.6% (95% CI: 3.8–18.3%) in 2006 to 13.8% (95% CI: 9.7–19.2%) in 2017 (p = 0.94).
Systemic risk factors
On multivariate analysis, factors associated with hospital admission included older age, specifically ≥81 years (odds ratio [OR] 32.59; [95% confidence interval (CI)]: 2.95–359.78]), and patients with the following comorbidities: pneumonia (OR 9.64 [95% CI: 1.25–74.35]), IVDU (OR 14.90 [95% CI: 1.67–133.16]), and renal/urinary tract infections (OR 4.09 [95% CI: 1.77–9.48]), (Table 3). Factors associated with mortality included HIV/immune deficiencies (OR 2.58 [95% CI: 1.26–5.28]), heart failure (OR 2.12 [95% CI: 1.47–3.05]), pneumonia (OR 1.64 [95% CI: 1.17–2.29]), renal/urinary tract infections (OR 1.87 [95% CI: 1.18–2.79]), and hepatic infections/cirrhosis (OR 1.89 [95% CI: 1.28–2.79]). DM was associated with a decreased risk for mortality (OR 0.49 [95% CI: 0.33–0.73]), (Table 3).
Discussion
There have been few multi-centre investigations examining EE across all regions of the country [15,16,17]. Previous studies have involved other databases utilizing Medicare insurance billing data for all inpatients (not patients through the emergency department), such as the National Inpatient Sample, but have not evaluated risk factors for mortality or admission [17]. This analysis evaluates risk factors associated with admission and mortality for EE using a large national database evaluating patients presenting to the ED. While the subgroup of IVDU-associated EE has recently been identified as increasing in incidence, our analysis shows that EE at-large has increased regardless of the underlying source [12]. Additionally, multiple comorbidities each demonstrated varying associations with admission or mortality in the setting of EE. These data may be useful in identifying those at higher risk and guiding management early in their clinical presentation.
Incidence rates for EE has been increasing, and the rise in IVDU only explains a subset of this change [12]. We presume a component of this elevation can be attributed to the overall rise of sepsis by 8.7% over the past two decades [18]. Explanations for growing sepsis rates include the increasing average age of the US population, thereby also increasing susceptibility to more comorbidities and predisposition to systemic infections [19, 20]. Additionally, there has been a greater use of antibiotics resulting in higher rates of antibiotic-resistant infections that also increases the rates of systemic infections [21]. Furthermore, with new advances in medicine, including immunotherapy, chemotherapy, and organ transplantation, there are increasing numbers of patients who are immunocompromised and more susceptible to systemic infections [22]. Finally, recent guidelines have increased surveillance for both sepsis and endophthalmitis, and not necessarily with improved outcomes [5, 6]. Hyperattentive screening paradigms could be contributing to the overdiagnosis of EE in those with non-specific retinal findings (e.g. Roth spots, cotton wool spots, haemorrhages) explained by underlying comorbidities, rather than infection from the bloodstream [6, 23].
The median inflation-adjusted cost of ED visits for EE increased over 11 years, although without statistical significance. A prior study by Friedman et al., evaluated mean inflation-adjusted charge for ED visits for all eye complaints using the NEDS database and found the cost was $1266 for emergent visits and $631 for nonemergent visits; these emergency visit expenses appear comparable to those in our study found with EE [24]. Although the study only compared visits from 2006 to 2011, an increase of $36/visit per year was identified, consistent with the non-significant increase in median cost we noted from 2006 to 2017 in our study [24]. A prior study evaluating EE in the setting of IVDU using the National Inpatient Sample, a large Medicare database, noted the median inflation-adjusted cost per hospitalization was $13,560 which also increased over recent years [15]. This expenditure reflects the entire hospital course in contrast with our analysis which accounts only for services rendered during the ED visit. Therefore, it remains important in future studies to determine the cumulative financial burden of EE that extends beyond the ED, and including the respective hospital course, as well as any surgery, follow-up and other medical services following the initial evaluation and management for complications from this disease.
The mortality rate in our study was 12.9%. Other studies evaluating mortality outcomes in patients with EE have noted a range from as low as 4%, to rates closer to our findings at 10% [2, 8]. Studies that cite lower numbers such as 4% by Jackson’s group included only patients with bacterial EE and excluded patients with fungal EE (shown to have higher mortality rates, as high as 30%) [2, 6, 25]. Higher rates in our study can be explained by inclusion of both bacterial and fungal EE as well as the inherent bias of using a database derived from ED visits. Patients with EE presenting to the ED might present at a later and more severe stage of the disease process in comparison to patients presenting to an ophthalmology clinic. Furthermore, the median age of our population was 62 which is older than the mean age of patients (52) in Jackson et al.’s study, and therefore our population may have been more susceptible to age-related comorbid conditions [2]. While statistically insignificant, the trend towards increased mortality over time suggests that the risk of mortality may be increasing. This finding could be attributed to the factors highlighted above including an aging population, increased use of immunotherapy, and emergence of novel, more virulent pathogens [25]. Regardless, optimizing systemic antimicrobial management for the septicaemia is imperative for treating EE as well as increasing odds for survival of these patients [6, 26].
HIV/immune deficiencies, heart failure, pneumonia, renal/urinary tract infections and hepatic infections/cirrhosis were associated with mortality with immune deficiency having the highest odds ratio for mortality. All of these conditions either result in primary dysfunction of the immune system or predispose individuals to unsustainable, high metabolic demands by which sepsis imposes on human physiology [27]. Studies that have examined mortality in patients with sepsis have noted that patients with primary infections of certain origins such as respiratory or gastrointestinal systems were more likely to have higher rates of organ dysfunction than patients with other sources of sepsis [28]. Furthermore, certain immune deficiencies predispose to septicaemia with particular organisms. For example, impaired neutrophil function predisposes to infection with Apergillus which portends a more severe course [11]. The compounding factor of immune dysfunction with vulnerability to certain pathogens can help explain why these comorbidities were identified to increase mortality [2, 29, 30]. Further investigation with specific analysis on microbiologic data can further help elucidate these associations.
The two risk factors in our study that increased both mortality and admission were the presence of pneumonia and renal/UTI comorbidities. These two risk factors were also noted to have high prognostic prediction for mortality in Weng et al.’s systemic analysis of endophthalmitis (which included both exogenous and endogenous causes) using a health care database from a Taiwanese population [31]. Although the exact mechanism to explain these associations has yet to be elucidated, there are several postulations to explain this association. Commonly isolated organisms with chronic dialysis include Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas (P.) aeruoginosa, and Serratia marcescens which may all be on the rise [23, 32]. In particular, dialysis patients were at higher risk for infection with P. aeruoginosa than a control group, and showed a more severe septicaemic course [33]. Furthermore, dialysis patients are more likely to have external vascular access which is also a risk factor for bacteremia [34]. Poor prognosis in patients with pneumonia can reflect the overall severity of the patient’s septicaemia, as septicaemia associated with respiratory tract infections has been shown to have higher mortality rates than other systemic comorbidities [28, 35].
In our study, IVDU and endocarditis were associated with increased rate of admission but did not confer an increased risk of mortality. Although we did not stratify the age of our cohort based on comorbidities, we presume these two risk factors did not confer an increased risk of mortality as patients who partake in IVDU and develop septicaemia as a consequence tend to be younger. This concept is supported by several case series examining endophthalmitis with IVDU; the mean age in both these series were approximately mid-30s in years and had no reported mortality [36, 37].
DM here was associated with a decrease in mortality. This observation has been noted both in studies evaluating endophthalmitis and in studies looking at mortality trends in patients with sepsis [31, 38]. The mechanism to explain this association remains unclear. Studies have compared coagulation pathways and expression of inflammatory cytokines between patients with DM and patients without DM during sepsis, but no difference between the two cohorts have been identified [39,40,41,42]. One hypothesis possibly explaining the improved outcomes in patients with DM is the immune response to hyperglycaemia. Patients with DM chronically experience hyperglycaemia, and appear to have a decreased inflammatory response in this setting; in patients without DM, short term hyperglycaemia is an independent risk factor for mortality from significantly increased cytokine levels in response to this elevated glucose [43,44,45,46]. Furthermore, DM diminishes immune function by affecting multiple pathways including polymorphonuclear leukocyte migration and activation, and downregulation of signalling pathways involved in the immune system [47,48,49,50]. It is unclear how these multimodal effects of DM affect patients during sepsis and thus warrant further investigation.
Although this study drew from a large, nationally representative sample for a relatively rare disease process, there are inherent limitations from using a database. First, since the NEDS database is derived from coding in medical records, it is susceptible to missing data, errors in documentation, and unverified clinical findings. Furthermore, there is not a specific diagnostic code for EE. We derived our sample size from including patients with both a diagnosis of septicaemia and endophthalmitis. By eliminating patients without both of those diagnoses, we might have missed some patients in our cohort, and furthermore we might have erroneously included patients with a diagnosis of septicaemia and endophthalmitis not related to the sepsis if patients concurrently had both diagnoses. However, our approach in requiring two distinct diagnostic codes (specifically endophthalmitis and sepsis) was expected to add rigor, and one that buffers the robustness of this analysis, while minimizing the error of including patients who did not have EE. Another limitation inherent to the database is that each visit represented one admission and patients were not able to be followed longitudinally. Therefore, patients who are discharged and return to the ED could be represented more than once, and patients who passed away from EE but after discharge were not included in the mortality data in this study. Next, the database focuses on systemic variables and does not include more granular data such as ophthalmic function (e.g. visual acuity), microbiological or other clinical data to confirm diagnosis of EE, or severity of illness and presentation limiting some of the outcomes and interpretations that can be drawn. Since the database includes patients who first present to the ED it might not be representative of patients who are diagnosed with EE after being admitted in the hospital for sepsis. Finally, hospitals without an ophthalmology service in their hospital might have missed cases of EE.
Conclusion
EE, regardless of underlying source, has recently increased in incidence throughout the US. Explanations for this increase include an aging population, advances in medical care increasing the numbers of immunocompromised individuals, and hyperattentive screening paradigms. The two systemic factors that conferred both an increase in mortality and admission were pneumonia, and renal/UTI. These factors predispose to infections with more virulent organisms and have been shown to act as independent markers for worse prognosis in patients with sepsis. Finally, DM was shown to be associated with decreased mortality, which has also been identified in other studies evaluating both sepsis and endophthalmitis. This association warrants further investigation to elucidate how glycaemic regulation affects immune function and has the potential to influence how glucose is managed in patients with sepsis during times of such high metabolic demand. In conclusion, EE will continue to present as an increasing diagnostic and therapeutic challenge; identification of patient demographics, prognostic factors and visit characteristics may serve to improve current management paradigms.
Summary table
What was known before
-
Endogenous endophthalmitis (EE) comprises approximately 2–8% of all cases of endophthalmitis.
-
The eye becomes a secondary site of inoculation with hematogenous spread of infection from elsewhere.
What this study adds
-
EE has increased in incidence throughout US.
-
The two systemic factors that conferred both an increase in mortality and admission were pneumonia, and renal/UTI.
-
Additional exploration of the potential protective association of DM with decreased mortality in this context is needed.
Data availability
The data that support the findings of this study are available from the Nationwide Emergency Department Sample (NEDS) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of NEDS.
References
Novosad BD, Callegan MC. Severe bacterial endophthalmitis: towards improving clinical outcomes. Expert Rev Ophthalmol. 2010;5:689–98.
Jackson TL, Paraskevopoulos T, Georgalas I. Systematic review of 342 cases of endogenous bacterial endophthalmitis. Surv Ophthalmol. 2014;59:627–35.
Greenwald MJ, Wohl LG, Sell CH. Metastatic bacterial endophthalmitis: a contemporary reappraisal. Surv Ophthalmol. 1986;31:81–101.
Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: A 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48:403–23.
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409–17.
Breazzano MP, Day HR Jr, Bloch KC, Tanaka S, Cherney EF, Sternberg P Jr, et al. Utility of ophthalmologic screening for patients with Candida Bloodstream Infections: a systematic review. JAMA Ophthalmol. 2019;137:698–710.
Ratra D, Saurabh K, Das D, Nachiappan K, Nagpal A, Rishi E, et al. Endogenous endophthalmitis: A 10-Year Retrospective Study at a Tertiary Hospital in South India. Asia Pac. J Ophthalmol (Philos). 2015;4:286–92.
Hsieh M-C, Chen S-N, Cheng C-Y, Li K-H, Chuang C-C, Wu J-S, et al. Clinicomicrobiological profile, visual outcome and mortality of culture-proven endogenous endophthalmitis in Taiwan. Sci Rep. 2020;10:12481.
Schiedler V, Scott IU, Flynn HW Jr, Davis JL, Benz MS, Miller D. Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. Am J Ophthalmol. 2004;137:725–31.
Essman TF, Flynn HW Jr, Smiddy WE, Brod RD, Murray TG, Davis JL, et al. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg lasers. 1997;28:185–94.
Sridhar J, Flynn HW Jr, Kuriyan AE, Miller D, Albini T. Endogenous fungal endophthalmitis: risk factors, clinical features, and treatment outcomes in mold and yeast infections. J Ophthalmic Inflamm Infect. 2013;3:60.
Budoff G, Thangamathesvaran L, Zarbin MA, Bhagat N. Bacterial endogenous endophthalmitis in bacteremic inpatients. Ophthalmol Retin. 2019;3:971–8.
Uppuluri A, Zarbin MA, Budoff G, Bhagat N. Risk factors for endogenous endophthalmitis in hospitalized patients with Candida Fungemia. Ophthalmol Retin. 2021;5:687–95.
NEDS Database Documentation. Healthcare Cost and Utilization Project (HCUP) [Internet]. Agency for Healthcare Research and Quality. 2020. Available from: www.hcup-us.ahrq.gov/db/nation/neds/nedsdbdocumentation.jsp.
Mir TA, Papudesu C, Fang W, Hinkle DM. Incidence of drug use–related endogenous endophthalmitis hospitalizations in the United States, 2003 to 2016. JAMA Ophthalmol. 2021;139:18–26.
Papudesu C, Mir T, Fang W, Thompson J, Hinkle DM. Trends in infantile endogenous endophthalmitis hospitalizations in the United States: an analysis from 2007 through 2014 using the national inpatient sample. Ophthalmol Retin. 2020;4:1109–17.
Vaziri K, Pershing S, Albini TA, Moshfeghi DM, Moshfeghi AA. Risk factors predictive of endogenous endophthalmitis among hospitalized patients with hematogenous infections in the United States. Am J Ophthalmol. 2015;159:498–504.
Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl J Med. 2003;348:1546–54.
Shiels MS, Almeida JS, García-Closas M, Albert PS, Freedman ND, Berrington, et al. Impact of population growth and aging on estimates of excess U.S. deaths during the COVID-19 pandemic, March to August 2020. Ann Intern Med. 2021;174:437–43.
Chee SP, Jap A. Endogenous endophthalmitis. Curr Opin Ophthalmol. 2001;12:464–70.
Pradipta IS, Sodik DC, Lestari K, Parwati I, Halimah E, Diantini A, et al. Antibiotic resistance in sepsis patients: evaluation and recommendation of antibiotic use. N. Am J Med Sci. 2013;5:344–52.
Kalil AC, Opal SM. Sepsis in the severely immunocompromised patient. Curr Infect Dis Rep. 2015;17:487.
Breazzano MP, Bond JB 3rd, Bearelly S, Kim DH, Donahue SP, Lum F, et al. American Academy of Ophthalmology recommendations on screening for endogenous candida endophthalmitis. Ophthalmology. 2022;129:73–6.
Channa R, Zafar SN, Canner JK, Haring RS, Schneider EB, Friedman DS. Epidemiology of eye-related emergency department visits. JAMA Ophthalmol. 2016;134:312–9.
Breazzano MP, Tooley AA, Godfrey KJ, Iacob CE, Yannuzzi NA, Flynn HW. Candida auris and endogenous panophthalmitis: clinical and histopathological features. Am J Ophthalmol Case Rep. 2020;19:100738.
Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of <em>Candida</em> bloodstream infection until positive blood culture results are obtained: a potential risk factor for Hospital Mortality. Antimicrobial Agents Chemother. 2005;49:3640–5.
Arfaras-Melainis A, Polyzogopoulou E, Triposkiadis F, Xanthopoulos A, Ikonomidis I, Mebazaa A, et al. Heart failure and sepsis: practical recommendations for the optimal management. Heart Fail Rev. 2020;25:183–94.
Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;34:2576–82.
Bhavsar MM, Devarajan TV, Nembi PS, Ramakrishnan N, Mani AK. Metastatic endogenous endophthalmitis: a rare presentation with methicillin-resistant staphylococcus aureus prostatic abscess. Indian J Crit Care Med: peer-reviewed, Off Publ Indian Soc Crit Care Med. 2017;21:172–5.
Sadiq MA, Hassan M, Agarwal A, Sarwar S, Toufeeq S, Soliman MK, et al. Endogenous endophthalmitis: diagnosis, management, and prognosis. J Ophthalmic Inflamm Infect. 2015;5:32.
Weng T-H, Chang H-C, Chung C-H, Lin F-H, Tai M-C, Tsao C-H, et al. Epidemiology and mortality-related prognostic factors in endophthalmitis. Investigative Ophthalmol Vis Sci. 2018;59:2487–94.
Breazzano MP, Jonna G, Nathan NR, Nickols HH, Agarwal A. Endogenous Serratia marcescens panophthalmitis: a case series. Am J Ophthalmol Case Rep. 2019;16:100531.
Kuo G, Lu Y-A, Sun W-C, Chen C-Y, Kao H-K, Lin Y, et al. Epidemiology and outcomes of Endophthalmitis in chronic dialysis patients: a 13-year experience in a tertiary referral center in Taiwan. BMC Nephrol. 2017;18:270.
Stevenson KB, Hannah EL, Lowder CA, Adcox MJ, Davidson RL, Mallea MC, et al. Epidemiology of hemodialysis vascular access infections from longitudinal infection surveillance data: predicting the impact of NKF-DOQI clinical practice guidelines for vascular access. Am J Kidney Dis: Off J Natl Kidney Found. 2002;39:549–55.
Rhee C, Jones TM, Hamad Y, Pande A, Varon J, O’Brien C, et al. Prevalence, underlying causes, and preventability of sepsis-associated mortality in US Acute Care Hospitals. JAMA Netw Open. 2019;2:e187571–e.
Connell PP, O’Neill EC, Amirul Islam FM, Buttery R, McCombe M, Essex RH, et al. Endogenous endophthalmitis associated with intravenous drug abuse: seven-year experience at a tertiary referral center. Retin (Phila, Pa). 2010;30:1721–5.
Tirpack AR, Duker JS, Baumal CR. An outbreak of endogenous fungal endophthalmitis among intravenous drug abusers in New England. JAMA Ophthalmol. 2017;135:534–40.
Wang Z, Ren J, Wang G, Liu Q, Guo K, Li J. Association between diabetes mellitus and outcomes of patients with sepsis: a meta-analysis. Med Sci Monit: Int Med J Exp Clin Res. 2017;23:3546–55.
Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109:2698–704.
Annane D, Bellissant E, Cavaillon J-M. Septic shock. Lancet. 2005;365:63–78.
Kinasewitz GT, Yan SB, Basson B, Russell JA, Cariou A, Um SL, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569]. Crit Care. 2004;8:R82.
Stegenga ME, Vincent J-L, Vail GM, Xie J, Haney DJ, Williams MD, et al. Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit Care Med. 2010;38:539–45.
Krinsley JS, editor Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clinic Proceedings; 2003: Elsevier.
Leonidou L, Mouzaki A, Michalaki M, DeLastic AL, Kyriazopoulou V, Bassaris HP, et al. Cytokine production and hospital mortality in patients with sepsis-induced stress hyperglycemia. J Infect. 2007;55:340–6.
Jiménez-Ibáñez EO, Castillejos-López M, Hernández A, Gorocica P, Alvarado-Vásquez N. High mortality associated with hyperglycemia, neutrophilia, and lymphopenia in critically ill patients. Tohoku J Exp Med. 2012;226:213–20.
Yu W-K, Li W-Q, Li N, Li J-S. Influence of acute hyperglycemia in human sepsis on inflammatory cytokine and counterregulatory hormone concentrations. World J Gastroenterology: WJG. 2003;9:1824.
Venot M, Weis L, Clec’h C, Darmon M, Allaouchiche B, Goldgran-Tolédano D, et al. Acute kidney injury in severe sepsis and septic shock in patients with and without diabetes mellitus: a multicenter study. PLoS ONE. 2015;10:e0127411.
Valerius N, Eff C, Hansen N, Karle H, Nerup J, Søeberg B, et al. Neutrophil and lymphocyte function in patients with diabetes mellitus. Acta Med Scandinavica. 1982;211:463–7.
Masuda M, Murakami T, Egawa H, Murata K. Decreased fluidity of polymorphonuclear leukocyte membrane in streptozocin-induced diabetic rats. Diabetes. 1990;39:466–70.
Cui Y, Chen W, Chi J, Wang L. Comparison of transcriptome between type 2 diabetes mellitus and impaired fasting glucose. Med Sci Monit: Int Med J Exp Clin Res. 2016;22:4699.
Funding
This research was supported by unrestricted grants including Research to Prevent Blindness to the Wilmer Eye Institute (Baltimore, MD, USA) as well as from the VitreoRetinal Surgery Foundation in Edina, MN, USA (LT, MPB) and the Kogod Family (MPB).
Author information
Authors and Affiliations
Contributions
Each author contributed to the manuscript as follows: design and conduct of the study (LT, JC, AWS, FAW, MPB); collection, management, analysis, and interpretation of the data (LT, JC, MPB); preparation, review, or approval of the manuscript and decision to submit for publication (LT, JC, AWS, FAW, MPB).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thangamathesvaran, L., Canner, J.K., Scott, A.W. et al. National emergency department trends for endogenous endophthalmitis: an increasing public health challenge. Eye 37, 1123–1129 (2023). https://doi.org/10.1038/s41433-022-02080-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02080-9
This article is cited by
-
Operative Therapie und Keimnachweis bei endogener Endophthalmitis
Die Ophthalmologie (2024)