Abstract
Purpose
To establish the factors responsible for attenuation of the optical coherence tomography (OCT) signal beneath the neurosensory detachment (NSD).
Methods
We retrospectively reviewed 33 eyes with acute central serous chorioretinopathy. The thickness of the neurosensory retina, the thickness of the photoreceptor outer segment (PROS) layer, the height of the NSD, and the reflectivity of the underlying retinal pigment epithelium (RPE) were measured at selected points of B-scans exported from 6 × 6 mm OCT angiography protocols. The intensity of the flow signal was measured at the corresponding regions of the choriocapillaris slab. The correlation between the parameters of the NSD and both the reflectivity of underlying RPE and the intensity of the flow signal in the choriocapillaris was calculated.
Results
Correlation coefficients between RPE reflectivity and neurosensory retinal thickness, PROS layer thickness, and NSD height were −0.32, −0.64, and −0.25, respectively (p < 0.001). Correlation coefficients between the intensity of the flow signal and neurosensory retinal thickness, PROS layer thickness, and NSD height were −0.24 (p = 0.004), −0.52 (p < 0.001), and 0.13 (p = 0.13), respectively.
Conclusion
The thickness of the PROS layer is the most significant factor affecting OCT signal at the level of RPE and OCT angiography flow signal in the choriocapillaris beneath the NSD.
Similar content being viewed by others
Introduction
Optical coherence tomography angiography (OCTA) has enabled a better understanding of retinal disorders by providing detailed information on retinal and choriocapillaris microcirculation. The latter, in particular, was not available for clinical evaluation before the introduction of the OCTA [1]. Additionally, high resolution and the absence of dye leakage have made detailed visualization and quantitative characterization of choroidal neovascularization (CNV) available [2, 3].
However, due to various artifacts which affect the resultant image, the interpretation of OCTA requires particular care. These artifacts include, but are not limited to: motion artifacts, shadowing artifacts, segmentation errors, suspended scattering particles in motion, and blood flow velocity outrange [4]. Some of these have an impact on OCTA imaging of the choriocapillaris since the scanning beam, before it reaches the choriocapillaris, also interacts with neurosensory retina and retinal pigment epithelium (RPE).
It has been previously shown that changes in overlying tissues affect the OCTA flow signal from the choriocapillaris [4]. This may be particularly important for the evaluation of CNV and perfusion of the choriocapillaris. One of the most frequent phenomena coexisting with CNV is neurosensory detachment (NSD). Although the decrease of the flow signal in the choriocapillaris has been described beneath the NSD [4], it is still not known how OCTA images of the choriocapillaris should be interpreted with respect to the morphology of the detached retina.
Here we aim to establish a correlation between the parameters of NSD and the optical coherence tomography (OCT) signal beneath the detached neurosensory retina assessed through measuring the RPE reflectivity and flow signal intensity in the choriocapillaris. In this study, we have included eyes with acute central serous chorioretinopathy (CSC). Acute CSC was chosen as a model for studying OCT signal changes since NSD is a common sign of CSC and because other parameters and factors, such as RPE reflectivity and optical media clarity, are relatively consistent in the cohort of acute CSC eyes.
Methods
The study followed the ethical standards stated in the Declaration of Helsinki and was approved by the Local Ethics Committee. Informed consent was obtained from all participants. This was a retrospective analysis of OCT and OCTA data of acute CSC patients from a local database. For each patient, only a single 6 × 6 mm OCTA scan for one eye was included in the analysis. All OCTA scans were obtained with the RTVue-XR Avanti (Optovue, Fremont, CA) running software version 2017.1.0.150. Exclusion criteria were OCTA scan quality score less than Q8, any coexistent ocular disorders, any refractive error more than 2.0 D, any history of laser treatment of retinal disorders, or history of cataract or refractive surgery. From every OCTA scan selected for analysis, an experienced retina specialist extracted 2–4 random b-scans at different positions all within the NSD, excluding scans crossing the leak and any RPE abnormalities or areas of choroidal hypertransmissive tracks.
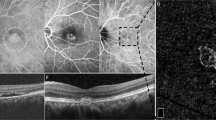
A masked grader uploaded the selected B-scans in ImageJ software and chose 1–6 points at random within the NSD on each scan. The number of points chosen for analysis per scan was dependent on the area occupied by NSD on this scan. In each selected point (1) neurosensory retinal thickness; (2) photoreceptor outer segment (PROS) layer thickness; (3) NSD height; and (4) RPE reflectivity were measured. Additionally, for every scan, a reference RPE reflectivity was measured at the temporal part of the scan outside the NSD. The neurosensory retinal thickness was defined as the distance from the inner limiting membrane to the outer border of the PROS layer. PROS layer thickness was defined as the distance from the inner border of the ellipsoid to the outer border of the PROS layer. NSD height was defined as the distance from the outer border of the PROS layer to the inner border of the RPE. All measurements were taken parallel to the direction of the scanning beam. RPE reflectivity was measured within the area 5 × 5 pixels exactly under the point where the measurements of neurosensory retina and NSD were made. Reference RPE reflectivity was measured in the representative 5 × 5 pixels area. The normalized RPE reflectivity was calculated as the ratio of the RPE reflectivity at the point of the measurements to the reference RPE reflectivity (Fig. 1).
The white lines represent neurosensory retinal thickness excluding photoreceptor outer segment layer. The black lines represent thickness of photoreceptor outer segment layer. The white dashed lines represent the height of neurosensory detachment. The white and black squares represent the areas of measuring of the retinal pigment epithelium reflectivity within neurosensory detachment and at the normal region, respectively.
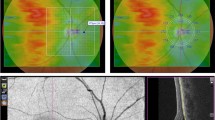
The default choriocapillaris slab was taken to analyse the correlation between the parameters of the detached retina and flow signal intensity. The flow signal intensity was measured as the mean gray value within the 25 × 8 pixels rectangular area placed along the selected b-scan. Neurosensory retinal thickness, PROS layer thickness, and NSD height were then measured at the corresponding point on the b-scan (Fig. 2). To exclude between-subject differences of the flow signal, we also calculated the normalized flow signal intensity as a ratio of the actual flow signal intensity to reference flow signal intensity (calculated as the mean flow signal intensity outside the NSD).
The white lines represent neurosensory retinal thickness excluding photoreceptor outer segment layer. The black lines represent thickness of photoreceptor outer segment layer. The white dashed lines represent the height of neurosensory detachment. The horizontal white dashed line represents the position of the B-scan. White boxes represent areas of evaluation of the flow signal, corresponding to measurements on the B-scan.
Statistical analysis was performed with MedCalc 18.4.1 (MedCalc Software, Otend, Belgium). Sample size was calculated by using a power 95% and a significance level of 5%. With a hypothesized correlation coefficient of 0.5, the minimal required number of analysed points was found to be 46. Correlation coefficient was calculated between both RPE reflectivity and choriocapillaris flow signal intensity and each parameter of the NSD. Additionally, the correlation was calculated for the RPE reflectivity and neurosensory retinal thickness without PROS layer. Multivariable regression models, which included all parameters of the NSD, were constructed for absolute RPE reflectivity and absolute flow signal intensity. P < 0.05 was considered statistically significant.
Results
We retrospectively reviewed 33 eyes of 33 patients (33 eyes of 30 males and 3 females with a mean age of 40.2 ± 4.9 years) with acute CSC (mean duration of symptoms 2.1 ± 1.7 months). In total, 89 b-scans (1–6 b-scans from each eye, with mean 2.5 ± 1.6 scans) were selected, and 289 points (1–6 points in each b-scan, with mean 3.5 ± 1.6 points) were analysed. At each of these points, the neurosensory retinal thickness, PROS thickness, NSD height, and RPE reflectivity were measured. To analyse the effect of the parameters of the detached retina on the flow signal intensity in the choriocapillaris, 63 b-scans (1–5 b-scans from each eye, with mean 2.0 ± 1.2 scans) were selected to analyse 69 points (1–5 points in each b-scan, with mean 2.1 ± 1.0 points). The number of points evaluated for choriocapillaris flow was less than that for RPE reflectivity since larger areas are evaluated in the analysis of choriocapillaris flow and in the same length of scan occupied by NSD, fewer points can be subjected to evaluation.
RPE reflectivity
Mean RPE reflectivity under the NSD and within the normal regions was 166.9 ± 25.1 and 206.4 ± 13.6, respectively (p < 0.001). The mean normalized RPE reflectivity under the NSD was 0.80 ± 0.12.
Correlation coefficients between RPE reflectivity and neurosensory retinal thickness, PROS thickness, and NSD height were −0.32, −0.64, and −0.25, respectively (p < 0.0001 for all parameters). However, there was no statistically significant correlation between the thickness of the neurosensory retina without PROS layer and RPE reflectivity (r = −0.02, p = 0.72) (Fig. 3).
Correlation coefficients between normalized RPE reflectivity and neurosensory retinal thickness, PROS thickness, and NSD height were −0.2 (p = 0.004), −0.54 (p < 0.001), and −0.21 respectively (p = 0.002). There was no statistically significant correlation between the thickness of the neurosensory retina without PROS and RPE reflectivity (r = −0.03, p = 0.70).
The multiple regression model, including neurosensory retinal thickness, PROS layer thickness, and NSD height, confirmed that the PROS layer thickness and NSD height correlate independently with RPE reflectivity (β = − 0.64, p < 0.001 and β = −0.44, p < 0.001, respectively).
Flow signal intensity
Correlation coefficients between the intensity of the flow signal and neurosensory retinal thickness and PROS thickness were −0.25 (p = 0.004) and −0.52 (p < 0.001), respectively (Fig. 3) (Fig. 4). However, there was no statistically significant correlation between the intensity of the flow signal and the thickness of the neurosensory retina without PROS layer (r = −0.1 p = 0.27) or NSD height (r = 0.13, p = 0.13).
Correlation coefficients between the normalized intensity of the flow signal and neurosensory retinal thickness and PROS thickness were −0.26 (p = 0.01) and −0.45 (p < 0.0001), respectively. However, there was no statistically significant correlation between the normalized intensity of the flow signal and the thickness of the neurosensory retina without PROS layer (r = −0.15, p = 0.15) or NSD height (r = 0.03, p = 0.76).
The multiple regression model, including neurosensory retinal thickness, PROS layer thickness, and NSD height confirmed that only the PROS layer thickness correlates independently with the intensity of the flow signal (β = −0.47, p < 0.001).
Discussion
In this study, we used RPE reflectivity as an indicator of changes in the OCT signal caused by the NSD. If no changes in OCT signal were caused by the detached retina, RPE reflectivity should demonstrate no, or minimal changes through the entire b-scan. However, the mean value of RPE reflectivity within the area of the NSD was substantially lower than that in normal regions, which is caused by absorption or scattering of the scanning beam by the detached retina.
We found that, within the NSD, the most significant structure affecting the OCT scanning beam is the PROS layer. Another important but less significant determinant is the height of the NSD: the higher the NSD, the more significant the OCT signal attenuation. Other retinal layers, except for the PROS layer, have minimal or no impact on the OCT signal.
It was observed that the OCTA signal from the choriocapillaris is significantly weaker within the area of the NSD [4]. Since subretinal fluid is generally transparent to the near-infrared light of the OCT scanning beam [5], only changes in the structure of the neurosensory retina may explain the attenuation of the flow signal observed beneath the detached retina. Indeed, between the attached and detached retina in CSC, it is the PROS layer which shows the most significant changes [6, 7]. In this study, the thickness of the PROS layer correlates significantly with the reflectivity of the underlying RPE. The flow signal intensity also demonstrated a correlation with the PROS layer thickness but not with other parameters of the NSD.
It is worth noting that after detachment of the retina, the PROS layer gets thicker, losses its arrangement, and looks shaggy and irregular [6, 7]. The latter changes of the PROS layer may also increase the scattering of the scanning beam and contribute to OCT signal attenuation by the thick PROS layer. Moreover, the PROS layer, even under normal conditions, is one of the most reflective structures of the neurosensory retina, which indicates its substantial interaction with the scanning beam. All of these may explain why the thickness of the PROS layer is the most significant determinant of OCT signal attenuation by the detached retina.
In our study, the height of the NSD was also found to be correlated with RPE reflectivity. This suggests that the weakening of the OCT signal depends not only on the PROS thickness but also on how far from the RPE the scanning beam interferes with the PROS layer. If we consider the PROS layer as the point where the scanning beam scatters, the higher the point of scattering above RPE, the larger the area covered by the scanning beam at the RPE and the lower the scanning beam intensity. Another reason why the height of the NSD contributes to OCT signal attenuation is the change in the direction of the scanning beam in relation to the PROS of the detached retina. When the retina is attached, the scanning beam is almost coaxial to the PROS, while with the detached retina, the scanning beam passes PROS at an angle, and the total length of the path through the PROS layer for the scanning beam increases. However, it is not fully understood why the NSD height does not affect the flow signal intensity.
The main application of the results of this paper lies in the interpretation of OCT/OCTA images in patients with NSD, specifically in age-related macular degeneration and CSC patients. This may include the evaluation of RPE integrity beneath the NSD. For example, normal RPE may appear altered if the PROS layer is thick but appears unchanged in the regions where the PROS layer is thin. The results of this study can be similarly applied to OCTA imaging. We, therefore, can expect that the flow signal on the OCTA image in the choriocapillaris slab may be distorted by irregularities of the PROS layer. Firstly, a local increase in the flow signal under PROS thinning may be recognized as CNV. Secondly, the reattachment of the neurosensory retina may change the appearance of the CNV depending on the status of the PROS layer. If the PROS layer is thick, CNV may be more clearly visible after the reattachment of the retina. In such cases, increased visualization of CNV may conflict with the resolution of subretinal fluid after the anti-VEGF treatment. Additionally, the fragments of a large CNV may be partially obscured if the PROS layer is irregular and may appear after the reattachment of the retina.
The main limitation of the study is that only CSC patients were included in the study cohort. Although NSD is a common phenomenon in age-related macular degeneration, measuring RPE reflectivity in elderly patients is challenging due to the high prevalence of media opacities and age-related RPE changes. CSC, therefore, seems a more attractive model for studying the effects of NSD on the OCT signal. Another limitation is the possible presence of undetectable general RPE alteration in CSC eyes dictated by CSC pathophysiology [8]. Some level of change of RPE reflectivity compared to healthy eyes, therefore, cannot be excluded, even in unaffected areas of the eye fundus of CSC eyes. From this point of view, measuring relative RPE reflectivity is more reliable. However, the data on relative RPE reflectivity completely agrees with that obtained for absolute RPE reflectivity.
In conclusion, if the neurosensory retina is detached, the PROS layer appears to be the main structure responsible for the reduction of the reflectivity of underlying RPE and the intensity of the flow signal in the choriocapillaris. This is likely caused by the attenuation of the OCT signal due to scattering and absorption of scanning beam by the PROS. We found that beneath the NSD, the thickness of the PROS layer was the most significant determinant for OCT signal attenuation, assessed as the RPE reflectivity, and the only determinant for the attenuation of the flow signal in the choriocapillaris.
Summary
What was known before:
-
There are various artifacts which affect the optical coherence tomography angiography imaging of choriocapillaris, including structural changes in overlying neurosensory retina and retinal pigment epithelium.
What this study adds:
-
The most significant structure affecting the optical coherence tomography signal beneath the neurosensory detachment, the photoreceptor outer segment layer.
-
Another important but less significant determinant is the height of the neurosensory detachment Among other parameters of the neurosensory retina, only the photoreceptor outer segment layer contributes to optical coherence tomography angiography signal attenuation in the choriocapillaris beneath neurosensory detachments.
References
Wang Q, Chan S, Yang JY, You B, Wang YX, Jonas JB, et al. Vascular density in retina and choriocapillaris as measured by optical coherence tomography angiography. Am J Ophthalmol. 2016;168:95–109.
Lumbroso B, Rispoli M, Savastano MC. Longitudinal optical coherence tomography–angiography study of type 2 naive choroidal neovascularization early response after treatment. Retina. 2015;35:2242–51.
de Carlo TE, Bonini Filho MA, Chin AT, Adhi M, Ferrara D, Baumal CR, et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology 2015;122:1228–38.
Kayat KV, Roisman L, Zett C, Novais EA, Farah ME. Choriocapillaris hypoperfusion artifact in OCT angiography. Ophthalmic Surg Lasers Imaging Retin. 2018;49:603–10.
van den Berg TJ, Spekreijse H. Near infrared light absorption in the human eye media. Vis Res. 1997;37:249–53.
Asano KS, Asaoka R, Asano S, Azuma K, Inoue T, Obata R. Elongated photoreceptor outer segment length and prognosis of chronic central serous chorioretinopathy. Retin. 2020;40:750–7.
Maltsev DS, Kulikov AN, Chhablani J. Topography-guided identification of leakage point in central serous chorioretinopathy: a base for fluorescein angiography-free focal laser photocoagulation. Br J Ophthalmol. 2018;102:1218–25.
Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retinal Eye Res. 2015;48:82–118.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maltsev, D.S., Kulikov, A.N. & Kazak, A.A. Photoreceptor outer segment layer contributes to optical coherence tomography signal attenuation beneath neurosensory detachments. Eye 36, 1795–1798 (2022). https://doi.org/10.1038/s41433-021-01736-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01736-2