Abstract
Purpose
To compare the time to resolution of perivascular infiltrates in tubercular retinal vasculitis (TRV) between anti-tubercular therapy (ATT) alone, and in combination with systemic corticosteroids.
Methods
Observational retrospective cohort study in a tertiary eye centre in eastern India. Patients with TRV who were treated with anti-tubercular therapy (ATT) alone (Group A), or in combination with systemic corticosteroids (Group B) were included in the study. Eyes with additional inflammatory signs (cystoid macular oedema, vitritis ≥2+, optic disc oedema) were excluded. Resolution was defined as complete disappearance of perivascular infiltrates on seven-field fundus photographs. Descriptive statistics were used for demographic data. A linear mixed effects model was applied to adjust for intereye correlations, in patients with bilateral disease. The primary outcome measure was time to resolution of perivascular infiltrates. Secondary outcome measure was need for laser or surgical intervention for management of complications of TRV.
Results
Fifty eyes of 39 patients (Group A 21/18 and Group B 29/21) were included. Both groups had similar demographics and severity of vasculitis. All patients had complete resolution of TRV. On adjusting for intereye correlation, the mean difference in time to resolution between the two groups (Group A, 3.24 [95% CI 2.69–3.77] months, and Group B, 4.76 [95% CI 3.52–5.99] months) was not statistically significant (0.96 weeks [−0.52 to 2.45] p = 0.21). Vaso-occlusive complications and healing patterns were similar in both groups.
Conclusions
ATT alone, may be sufficient for resolution of perivascular infiltrates, in TRV without additional inflammatory signs.
Similar content being viewed by others
Introduction
Anti-tubercular therapy (ATT) is central to the management of ocular tuberculosis (TB). Multiple studies have demonstrated the beneficial role of ATT, either through resolution of lesions on initiation of ATT, or through non-recurrence of inflammation after completion of treatment [1,2,3]. ATT is commonly used in combination with corticosteroids (local and/or systemic) in the management of ocular TB. However, the benefits of adjunctive corticosteroid therapy have remained inconclusive. A systematic review found no additional beneficial effect by concurrent use of corticosteroids [3], while a recent comparative study found no difference in visual or inflammatory outcomes on addition of systemic corticosteroids [4]. Not only have the benefits of corticosteroids been equivocal, but patients also run the additional risk of corticosteroid-induced adverse effects. Despite these limitations, corticosteroids continue to be used in management of ocular TB, ostensibly to control ocular inflammation, but also, we suspect, due to the lack of clarity on the pathogenesis of this condition.
The conundrum regarding adjunctive use of corticosteroids is best exemplified in the management of tubercular retinal vasculitis (TRV). TRV presents as perivascular infiltrates, typically involving veins in one or more quadrants [5]. Clinically, it is challenging to distinguish TRV from other forms of infectious and non-infectious retinal vasculitidis, and the diagnosis is typically made on the basis of ancillary evidence of latent or active systemic TB. This clinical dilemma leads to frequent use of adjunctive corticosteroids in management of TRV, as noted in the Collaborative Ocular Tuberculosis Study-1 (COTS-1) [6]. Recently, we have identified specific clinical signs that are predictive of tubercular aetiology in retinal vasculitis, in a TB-endemic country [7]. These include:
-
subvascular lesions (active or healed chorioretinitis lesions underlying blood vessels),
-
focal vascular tortuosity (FVT, localized tortuosity of retinal veins associated with healed or active perivascular exudates but away from areas of capillary non-perfusion),
-
occlusive vasculitis (presence of capillary non-perfusion in the drainage area of the affected vessel).
In the current study, we have reviewed patients in whom used these clinical predictors of TRV to initiate ATT, with or without adjunctive corticosteroids. We analysed the resolution of perivascular infiltrates, and patterns of healing in each group, to compare the time to resolution of TRV, between ATT alone, and ATT combined with systemic corticosteroids.
Methods
We conducted an observational retrospective cohort study in a tertiary eye centre in eastern India. We retrospectively reviewed electronic medical records of patients diagnosed as TRV between January 1, 2014 and June 30, 2019. The study was approved by the institutional review board and adhered to the tenets of Declaration of Helsinki. Active TRV was defined by presence of perivascular exudates associated with one or more of the following clinical signs: healed or active subvascular lesions, FVTs and/or signs of vascular occlusion; along with evidence of systemic tubercular infection (tuberculin skin test [TST], QuantiFERON TB Gold test [QFT], chest radiography) and/or positive polymerase chain reaction (PCR) for TB; and the exclusion of non-TB entities. Ocular imaging including fluorescein angiography (FA), optical coherence tomography and autofluorescence were performed at presentation, and thereafter during follow up, whenever necessary. We included patients who followed up for a minimum 6 months after initiation of ATT; and had complete documentation of clinical evaluation including seven-field montage images during the follow up. The exclusion criteria comprised of associated inflammatory signs that would usually require adjunctive local or systemic corticosteroid therapy. These included:
-
(1)
Vitritis ≥2+
-
(2)
Cystoid macular oedema (CMO)
-
(3)
Optic disc involvement (disc oedema or granuloma)
-
(4)
Retinitis lesions involving or threatening macula
Additional exclusion criteria were previous treatment with ATT for pulmonary or extra-pulmonary TB, or oral corticosteroids, and patients with prior vitrectomy or those requiring total vitrectomy prior to complete resolution of TRV. Patients who received topical corticosteroids for treatment of anterior uveitis were not excluded.
All patients received 6 months ATT for the treatment of TRV. These included 2 months of intensive therapy with isoniazid, rifampicin, ethambutol and pyrazinamide; and 4 months maintenance therapy with isoniazid and rifampicin. All doses were as per standard recommendations and were adjusted according to body weight. Oral vitamin B-complex was administered for pyridoxine supplementation. All patients were monitored by an internist for drug-related adverse effects. The dose of systemic corticosteroids, when used, and the tapering schedule, varied according to the degree of inflammation in the eye. Most patients treated with ATT alone, presented to us from 2018 onwards, when we started treating patients isolated retinal vasculitis (without other signs of severe intraocular inflammation) with ATT alone. The patients were divided into two groups. Group A consisted of patients who were treated with ATT alone; and Group B, of patients who received adjunctive systemic corticosteroid therapy. Resolution of TRV was defined by the complete disappearance of perivascular infiltrates on seven-field fundus photographs. Treatment failure was defined as progression of perivascular infiltrates despite treatment on two consecutive visits and/or two step increase in vitritis in any visit and/or development of other signs of inflammation such as disc oedema or chorioretinitis.
The primary outcome measure was the time to complete resolution of TRV in each group. Secondary outcome measures included the need for laser or surgical intervention for complications such as tractional retinal detachment (RD) or vitreous haemorrhage.
Statistical analysis
Descriptive statistics were used for demographic data. Normality of data was tested by the Kolmogorov–Smirnov test. Kaplan–Meier survival analysis curve with the log rank test was used to compare time of resolution between the two groups. A linear mixed effects model was applied to adjust for intereye correlation between two eyes of the same patient, for comparing time to resolution. Cox proportional hazard regression was used to compare the risk of persistence of inflammation. p value of <0.05 was considered statistically significant. The data were analysed using Statistical Package for Social Sciences (SPSS) version 21.0.
Results
We retrieved data of 156 eyes of 106 patients who received ATT for TRV during the study period. Among these, 50 eyes of 39 patients were finally included into the study based on inclusion and exclusion criteria. Majority (n = 58, 86.5%) of patients who were excluded, either had inadequate documentation of laboratory investigations, or had incomplete follow up. This happened due to the referral consultations at our clinic and patients following up locally, after reviewing with us. The distribution of patients in two groups and reasons for exclusion have been listed in the flowchart in Fig. 1. Group A comprised of 21 eyes of 18 patients while group B had 29 eyes of 21 patients. All patients received 6 months ATT. Major reasons for exclusion were inadequate photographic documentation or investigations, presence of CMO, vitritis ≥2+ and/or optic disc involvement, and follow up of <6 months after initiation of ATT.
The baseline demographic characteristics of each group are given in Table 1. Majority of cases were unilateral in both groups—15/18 (83.33%) in Group A and 13/21 (61.9%) in Group B. Age and gender were comparable between both groups, though males were predominant in each group (83.33% and 95.24%, respectively in A and B). The best-corrected visual acuity, duration of symptoms and systemic evidence of TB infection viz. radiological evidence of pulmonary/extra-pulmonary TB and positive TST or QFT tests, were also similar in each group. Vitreous biopsy for TB PCR was positive in three patients in each group. One patient in Group B was diagnosed only on basis of positive PCR for Mycobacterium tuberculosis. The associated clinical signs viz. subvascular lesions, FVTs and vascular occlusion, and the markers of severity of inflammation, namely anterior chamber cells, vitreous haze ≤ grade 1, were also comparable between both groups. However, there were more eyes with first order vessel involvement in Group B. All three clinical signs were present in 9 eyes in Group A, and 8 eyes in Group B; while at least two signs were present in 15 and 20 eyes, respectively. At least one of the three signs was present in all the eyes. Five patients in Group A and seven in Group B had pre-existing retinal neovascularisation (with vitreous haemorrhage and/or tractional RD) due to a prior episode of retinal vasculitis. The presenting vasculitis in all these eyes was away from the previous lesion. Finally, the severity of retinal vascular inflammation, as measured by the number of quadrants involved, and the incidence of first order vessel involvement, was also similar in each group. The mean follow up in Group A was 13.83 ± 14.13 months (median 8.5 months, range 6–54 months) and in Group B 25.14 ± 17.33 months (median 16 months, range 6–60 months). One patient in Group B developed non-itching, papular eruptions on the chest and back after 4 days of starting ATT. His oral steroids were continued while ATT was discontinued for 1 week with internist consult. After 1 week, weight-adjusted doses of ATT were restarted. No recurrence of skin eruption was reported till completion of ATT.
Treatment outcomes
We found complete primary resolution of perivascular infiltrates at final follow up, in all patients in both groups. The presenting and final visual acuities in each group have been compared in Fig. 2. On applying Student’s t test, the mean time to resolution for Group A, 3.24 (95% CI 2.69–3.77) months, was significantly less than Group B, 4.76 (95% CI 3.52–5.99) months (p = 0.047). However, on adjusting for intereye correlation when both eyes of the same patient were involved, the mean difference in time to resolution between the two groups was not statistically significant (0.96 weeks [−0.52 to 2.45] p = 0.21). Kaplan–Meier survival analysis curve with the log rank test between two groups showed resolution of 57.14% eyes at the end of 3 months in Group A while 51.72% eyes in Group B showed resolution during same time period (Fig. 3). By 6 months, all patients in Group A had resolved in contrast to 75.86% (n = 15) in Group B. By the last follow up, there was complete resolution in all eyes in both groups. The hazard ratio for persistence of inflammation in Group B, as compared to Group A, was 1.61 (95% CI 0.81–3.23, p = 0.17).
One patient in Group A with bilateral TRV, who had complete resolution in both eyes by 8 weeks, developed recurrent panuveitis in left eye at 4 months while on ATT, presenting as grade 2 vitritis, with disc oedema and anterior uveitis (2+ cells). There was no recurrence of retinal vasculitis in either eye. The patient was treated with posterior sub-Tenon’s triamcinolone acetonide along with tapering dose of topical and oral corticosteroids. The inflammation subsided completely in 4 weeks, and there was no recurrence till 6 months follow up. None of the other eyes had any recurrence of inflammation during the follow up period.
We also noted two patterns of resolution in the active subvascular retinitis lesions in either group (Fig. 4A–I). In the first pattern, active subvascular lesions, which were bright and yellowish, became dull in appearance along with resolution of surrounding periphlebitis (Fig. 4D, G). These lesions further became pigmented subvascular scars at around 2–3 months. In the second pattern, there was narrowing/obstruction of the blood vessel passing through the retinitis lesion, with development of a collateral vessel, connecting the proximal and distal parts of the obstructed vessel (Fig. 4E, H). Three eyes (all Group B) also had discrete retinitis lesions, not underlying a blood vessel, all of which resolved completely with treatment. Two eyes in Group B had branch retinal arteriolar occlusion with retinal whitening in the affected quadrant. These resolved with arteriolar narrowing and retinal thinning during the follow up period. In the FVTs, the vasculature typically straightened up during healing (Fig. 4F, I), though in some eyes, they continued to remain tortuous.
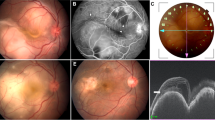
A–C Serial colour fundus photographs of right eye of a patient with tubercular retinal vasculitis (TRV) treated with anti-tubercular monotherapy at presentation, 2 weeks and 4 months post treatment, showing progressive resolution of perivascular infiltrates. Fundus photographs at presentation in TRV showing subvascular lesions (D, E, arrows) and focal vascular tortuosity (F, red free, inset). Corresponding fundus photographs at resolution showing two different patterns for subvascular lesions (G, H, arrows), and straightening of focal vascular tortuosity (I, red free, inset).
Eleven (52.38%) eyes in Group A and 14 (48.27%) eyes in Group B required sectoral laser photocoagulation for retinal or optic disc neovascularisation (p = 0.78), during the follow up period. The mean time from presentation to laser photocoagulation was 3.82 ± 1.8 months for Group A and 3.64 ± 2.92 months for Group B (p = 0.86). Four eyes in Group A and eight in Group B required pars plana vitrectomy for non-resolving vitreous haemorrhage and/or tractional RD. One patient in Group B also developed rhegmatogenous RD that required silicone oil injection. All these surgeries were performed after complete resolution of TRV in the respective eyes. None of the eyes in either group developed CMO during the follow up period.
Discussion
Our study demonstrates the therapeutic efficacy of ATT monotherapy in resolution of perivascular infiltrates in TRV. We found that the resolution of TRV in patients receiving monotherapy was comparable to those receiving adjunctive corticosteroids. ATT monotherapy also did not influence the incidence of long-term vaso-occlusive complications such as retinal or optic disc neovascularisation. Our results are tempered by the exclusion of eyes with additional ocular inflammatory signs at presentation such as dense vitritis, optic disc oedema and specifically, CMO. We also did not include a window period after completion of ATT for ruling out post-ATT recurrence, since we could directly observe the ATT-induced resolution of inflammation, without any confounding influences. Notwithstanding, our study convincingly demonstrates that ATT monotherapy can be sufficient for treatment of isolated TRV, without the need for adjunctive corticosteroids.
The role of corticosteroids in management of ocular TB has long been debated. A recent meta-analysis on the role of ATT in treatment of ocular TB showed that concurrent use of corticosteroids did not have any influence on treatment outcomes [3]. Individual studies too have shown that corticosteroid therapy prior to initiation of ATT is associated with poorer final outcomes [8, 9]. However, corticosteroids may be crucial in management of tubercular serpiginous-like choroiditis, both for the prevention and management of ATT-associated paradoxical worsening of lesions [2, 10]. Combination therapy with corticosteroids has also been found useful in certain forms of TRV [11]. Therefore, it is likely that adjunctive corticosteroids have varying roles in different clinical presentations of ocular TB.
In case of TRV, we believe that the use of corticosteroids is closely linked to our understanding of the pathomechanisms of the condition. The general perception about pathogenesis of TRV, though not stated commonly in published literature, is that TRV results from hypersensitivity to tuberculoproteins [12]. This has led to majority of physicians treating TRV with combination therapy, or even with corticosteroids alone. This was evident in the COTS-1 data from multiple geographic regions, where combined ATT and corticosteroid therapy was used 77.69% (195/251) of patients, while ATT alone was used in 13.14% (33/251) patients [6]. Seventeen (6.77%) patients received corticosteroids alone. However, we are increasingly beginning to appreciate the primary role of infection in pathogenesis of TRV. It has happened through histopathological studies [13,14,15], PCR studies [16,17,18] and clinical documentation of focal subvascular lesions in association with TRV [7, 16, 19]. This realisation led us to use ATT alone in a select subset of patients with TRV, where we obtained 100% therapeutic response. If TRV is driven by M. tuberculosis infection, one might speculate that corticosteroids would reduce the host immune response against the infection. However, at least in case of TB meningitis, adjunctive dexamethasone therapy was not found to have any influence on peripheral monocyte, or local T-cell responses, or on radiological appearance of lesions [20, 21]. The effect of corticosteroids on local immune response in ocular M. tuberculosis infection, remains unknown.
The therapeutic efficacy of ATT in the management of TRV, either alone or in combination with corticosteroids, has been documented earlier in several studies and case reports [16, 22,23,24,25]. Yet the COTS-1 study, could not draw a definite conclusion on the therapeutic benefit of ATT in TRV, likely due to small number of patients in the group that did not receive ATT [6]. Interestingly, none of the 33 patients who were treated with ATT alone in this study reported treatment failure, as against 15.89% (31/195) in the combination therapy and 17.65% (3/17) in the steroids alone groups. Treatment failure in this study was also more in the non-occlusive TRVs (though p = 0.09), and in Hispanics and African-Americans. The authors argued that while race could be a factor in the differential response to treatment, further studies are required to investigate the diagnostic criteria used for TRV in patients not responding to ATT. We agree with the authors’ point and believe that the presence of predictive clinical signs used for diagnosis of TRV in our study, facilitated the unequivocal therapeutic response in both the groups in our study. Future prospective studies comparing therapeutic response to ATT between eyes with and without the predictive clinical signs, will be able to further establish the utility of these signs.
Apart from its retrospective nature, our study was limited by the exclusion of patients with additional inflammatory signs such as CMO, dense vitritis and optic disc oedema. However, only seven patients (eight eyes, 7.5%) were excluded for the above reasons. Even in the COTS study, CMO and optic disc oedema/hyperaemia were found in only about 28%, and vitreous haze (of any grade) in 40% of all patients with TRV [6]. Thus, a significant proportion of TRV patients presenting to the clinic are likely to fit into the inclusion criteria of our study, and benefit from ATT monotherapy. Our change in strategy towards ATT monotherapy in 2018 could have also resulted in some degree of observer bias in favour of Group A. Therefore, a randomized control trial will be required to definitively demonstrate the value of ATT monotherapy in isolated TRV.
There was a strong potential of allocation bias in our study whereby more severe cases were likely to be treated with adjunctive corticosteroids. For example, there were more eyes with first order vessel involvement in Group B. However, the exclusion of patients with signs of severe inflammation (CMO, vitritis ≥2+ and disc oedema) from either group, reduced the possibility of such bias. The signs of severity of TRV (number of quadrants involved, and number of eyes with first order vessel involvement) were comparable between either group (Table 1). The mean follow up in Group A was less than Group B, and could have reduced the possibility of identifying post-treatment recurrences, though no recurrence was noted in either group.
We did not perform post-treatment FA to confirm complete resolution of vascular inflammation, as these patients present acutely, and resolve rapidly, following initiation of therapy. Notably, we did not find any influence on the healing pattern, or on the potential of vaso-occlusive complications such as retinal or optic disc neovascularisation, due to the absence of corticosteroids in the therapeutic regimen. Together, our study makes a strong case for ATT monotherapy in a select subset of TRV patients without additional inflammatory signs, and also highlights the primary role of infection in the pathogenesis of this condition.
Summary
What was known before
-
TB is a common cause of retinal vasculitis in TB-endemic countries.
-
TB-retinal vasculitis has characteristic clinical signs, at least in endemic countries.
What this study adds
-
Anti-TB therapy alone can cause resolution of perivascular infiltrates in TB-retinal vasculitis.
-
The resolution is faster compared to use of adjunctive corticosteroid therapy.
References
Bansal R, Gupta A, Gupta V, Dogra MR, Bambery P, Arora SK. Role of anti-tubercular therapy in uveitis with latent/manifest tuberculosis. Am J Ophthalmol. 2008;146:772–9. https://doi.org/10.1016/j.ajo.2008.06.011.
Basu S, Nayak S, Padhi TR, Das T. Progressive ocular inflammation following anti-tubercular therapy for presumed ocular tuberculosis in a high-endemic setting. Eye. 2013;27:657–62. https://doi.org/10.1038/eye.2013.5.
Kee AR, Gonzalez-Lopez JJ, Al-Hity A, Gupta B, Lee CS, Gunasekeran DV, et al. Anti-tubercular therapy for intraocular tuberculosis: a systematic review and meta-analysis. Surv Ophthalmol. 2016;61:628–53. https://doi.org/10.1016/j.survophthal.2016.03.001.
Nahon-Esteve S, Martel A, Maschi C, Alketbi M, Baillif S, Tieulie N. Uveitis associated with latent tuberculosis: a comparative study of the impact of antitubercular therapy combined or not with systemic corticosteroids. Eur J Ophthalmol. 2020:1120672120962066. https://doi.org/10.1177/1120672120962066.
Gupta V, Shoughy SS, Mahajan S, Khairallah M, Rosenbaum JT, Curi A, et al. Clinics of ocular tuberculosis. Ocul Immunol Inflamm. 2015;23:14–24. https://doi.org/10.3109/09273948.2014.986582.
Gunasekeran DV, Agrawal R, Agarwal A, Carreño E, Raje D, Aggarwal K, et al. The Collaborative Ocular Tuberculosis Study (COTS)-1: a multinational review of 251 patients with tubercular retinal vasculitis. Retina 2019;39:1623–30. https://doi.org/10.1097/IAE.0000000000002194.
Kaza H, Tyagi M, Pathangay A, Basu S. Clinical predictors of tubercular retinal vasculitis in a high endemic country. Retina. 2021;41:438–44. https://doi.org/10.1097/IAE.0000000000002829.
Hamade IH, Tabbara KF. Complications of presumed ocular tuberculosis. Acta Ophthalmol. 2010;88:905–9. https://doi.org/10.1111/j.1755-3768.2009.01579.x.
Patel SS, Saraiya NV, Tessler HH, Goldstein DA. Mycobacterial ocular inflammation: delay in diagnosis and other factors impacting morbidity. JAMA Ophthalmol. 2013;131:752–8. https://doi.org/10.1001/jamaophthalmol.2013.71.
Gupta V, Bansal R, Gupta A. Continuous progression of tubercular serpiginous-like choroiditis after initiating antituberculosis treatment. Am J Ophthalmol. 2011;152:857–63.e2. https://doi.org/10.1016/j.ajo.2011.05.004.
Agarwal M, Biswas J. Unilateral frosted branch angiitis in a patient with abdominal tuberculosis. Retin Cases Brief Rep. 2008;2:39–40. https://doi.org/10.1097/01.iae.0000247167.81281.53.
El-Asrar AM, Al-Kharashi SA. Full panretinal photocoagulation and early vitrectomy improve prognosis of retinal vasculitis associated with tuberculoprotein hypersensitivity (Eales’ disease). Br J Ophthalmol. 2002;86:1248–51. https://doi.org/10.1136/bjo.86.11.1248.
Verhoeff FH, Simpson GV. Tubercle within central retinal vein: haemorrhagic glaucoma; periphlebitis retinalis in other eye. Arch Ophthalmol. 1940;24:645.
Saini JS, Mukherjee AK, Nadkarni N. Primary tuberculosis of the retina. Br J Ophthalmol. 1986;70:533–5.
Basu S, Mittal R, Balne PK, Sharma S. Intraretinal tuberculosis. Ophthalmology. 2012;119:2192–3.e1.
Gupta A, Gupta V, Arora S, Dogra MR, Bambery P. PCR-positive tubercular retinal vasculitis: clinical characteristics and management. Retina. 2001;21:435–44.
Singh R, Toor P, Parchand S, Sharma K, Gupta V, Gupta A. Quantitative polymerase chain reaction for Mycobacterium tuberculosis in so-called Eales’ disease. Ocul Immunol Inflamm. 2012;20:153–7. https://doi.org/10.3109/09273948.2012.658134.
Verma A, Biswas J, Dhanurekha L, Gayathri R, Therese KL. Detection of Mycobacterium tuberculosis with nested polymerase chain reaction analysis in enucleated eyeball in Eales’ disease. Int Ophthalmol. 2016;36:413–7. https://doi.org/10.1007/s10792-015-0144-9.
Gupta A, Bansal R, Gupta V, Sharma A, Bambery P. Ocular signs predictive of tubercular uveitis. Am J Ophthalmol. 2010;149:562–70.
Fountain JA, Werner RB. Tuberculous retinal vasculitis. Retina 1984;4:48–50. https://doi.org/10.1097/00006982-198400410-00008.
Simmons CP, Thwaites GE, Quyen NT, Chau TT, Mai PP, Dung NT, et al. The clinical benefit of adjunctive dexamethasone in tuberculous meningitis is not associated with measurable attenuation of peripheral or local immune responses. J Immunol. 2005;175:579–90. https://doi.org/10.4049/jimmunol.175.1.579.
Thwaites GE, Macmullen-Price J, Tran TH, Pham PM, Nguyen TD, Simmons CP, et al. Serial MRI to determine the effect of dexamethasone on the cerebral pathology of tuberculous meningitis: an observational study. Lancet Neurol. 2007;6:230–6. https://doi.org/10.1016/S1474-4422(07)70034-0.
Shah SM, Howard RS, Sarkies NJ, Graham EM. Tuberculosis presenting as retinal vasculitis. J R Soc Med. 1988;81:232–3.
Doycheva D, Pfannenberg C, Hetzel J, Deuter CM, Pavesio C, Kempf VA, et al. Presumed tuberculosis-induced retinal vasculitis, diagnosed with positron emission tomography (18F-FDG-PET/CT), aspiration biopsy, and culture. Ocul Immunol Inflamm. 2010;18:194–9. https://doi.org/10.3109/09273948.2010.483318.
Agarwal A, Karkhur S, Aggarwal K, Invernizzi A, Singh R, Dogra MR, et al. Epidemiology and clinical features of inflammatory retinal vascular occlusions: pooled data from two tertiary-referral institutions. Clin Exp Ophthalmol. 2018;46:62–74.
Acknowledgements
The study was supported by Hyderabad Eye Research Foundation. We also thank Krishna Gopal Panda and Aniruddha Banerjee for fundus photography, Bhawna Garg for most statistical analyses, Mohammed Hasnat Ali for performing statistical analysis for intereye corelation.
Author information
Authors and Affiliations
Contributions
AK: Data collection and analysis; revision of manuscript; final approval. VG: Data collection and analysis; revision of manuscript; final approval. AK: Data collection and analysis; revision of manuscript; final approval. SB: Conception of study; analysis; initial draft and revision of manuscript. All authors approved the submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kelgaonkar, A., Govindhari, V., Khalsa, A. et al. Anti-tubercular therapy alone for treatment of isolated tubercular retinal vasculitis. Eye 36, 1777–1782 (2022). https://doi.org/10.1038/s41433-021-01727-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01727-3