Abstract
Purpose
To analyse choroidal vascular properties using an image binarization tool in patients with asymmetric pseudoexfoliative glaucoma (PXG) and compare them with healthy individuals.
Methods
This cross-sectional study included 144 eyes of 96 patients. The eyes were divided into three groups: 48 glaucomatous eyes and 48 non-glaucomatous contralateral eyes with no clinically observable pseudoexfoliation material of patients with asymmetric PXG, and 48 control eyes. Enhanced depth imaging optical coherence tomography scans of the macula and 3.4-mm diameter, 360-degree circle scans of the optic nerve head were binarized using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The choroidal vascularity index (CVI) was calculated as the ratio of the luminal area to the total circumscribed choroidal area.
Results
The macular CVI (mCVI) was significantly lower in the glaucomatous eyes than in the fellow eyes (p = 0.007) and the control eyes (p = 0.001). The peripapillary CVI (pCVI) in all sectors was significantly lower in the glaucomatous eyes than in the other two groups (all p < 0.05). Non-glaucomatous fellow eyes had lower CVI values in the macula and in the peripapillary region, except for the superior-nasal and nasal sectors, compared to the control eyes (all p < 0.05). In multivariate regression analysis, while the cup-to-disc ratio was negatively associated with the pCVI, AL was negatively associated with the mCVI in both eyes of patients with PXG.
Conclusions
CVI was decreased in the macula and peripapillary area in glaucomatous eyes. Furthermore, the CVI tended to decrease in non-glaucomatous fellow eyes of PXG patients. This finding may suggest subclinical involvement and require further exploration into the pathogenesis of glaucoma.
Similar content being viewed by others
Introduction
Glaucoma is a progressive optic neuropathy that causes loss of retinal ganglion cells (RGC) and their axons [1]. Although intraocular pressure (IOP) is the main risk factor, vascular dysfunction plays an important role in the etiopathogenesis of glaucoma [2]. Glaucoma involves comorbidity in vascular pathologies such as migraine, arterial hypertension and hypotension, low ophthalmic arterial blood pressure, and diabetes mellitus. Vascular dysfunction and insufficiency in the optic nerve head and retina can lead to ischaemia that contributes to RGC degeneration [3]. Reduced ocular perfusion is associated with the triggering, development, or progression of glaucomatous damage at the optic nerve head [4].
Pseudoexfoliative glaucoma (PXG) is the most common identifiable cause of open-angle glaucoma secondary to the accumulation of fibrillary pseudoexfoliative material (PXM) [5]. Pseudoexfoliative deposits have also been shown to accumulate in many extraocular structures other than the eye, such as the skin, heart, lung, kidneys, liver, gallbladder, urinary bladder, meninges, and vascular endothelium [6]. In fact, pseudoexfoliation syndrome (PXS) is a systemic disorder associated with vascular dysfunction. The predisposition to fibrin clot formation, increase in homocysteine levels, and endothelial dysfunction plays a role in vascular impairment [7].
Ocular involvement initially occurs unilaterally in approximately two-thirds of documented cases [8]. However, PXM was demonstrated by electron microscopy in up to 81% of unaffected contralateral eyes of clinically unilateral cases at the time of diagnosis [9]. In the Reykjavik Eye Study, 71% of unilateral PXS cases with a prospective 12-year follow-up record had converted to the bilateral form [10]. In addition, 32% of initially affected eyes and 38% of initially non-PXM contralateral fellow eyes had developed PXG by the 10-year follow-up visit [11]. Although PXM may not be clinically observed in both eyes, it is considered to be a condition with bilateral asymmetric involvement.
In an electron microscopic study, PXM has been found in the walls of the posterior ciliary arteries, vortex veins, and central retinal vessels in intraocularly unaffected fellow eyes [12]. Yüksel and colleagues detected increased resistive indices and reduced blood flow velocity in the retrobulbar vessels by colour Doppler imaging in PXS and PXG cases [13]. In addition, Dayanir and colleagues demonstrated these same hemodynamic alterations in the unaffected eyes of patients with clinically unilateral PXS [14]. In an optical coherence tomography (OCT) angiography study, the eyes with pseudoexfoliation exhibited significant changes in the retinal and choroidal vascular structures [15]. Li et al. found decreased macular thickness in patients with PXS and PXG [16].
The choroid is involved in the blood circulation of the prelaminar, laminar, and retrolaminar regions of the optic nerve head and the outer segments of the retina [17, 18]. The choroidal vascularity index (CVI) is a novel marker representing the vascular microarchitecture of the choroid [19, 20]. The choroidal structure is viewed as a luminal area (LA) and a stromal area (SA) using the image binarization tool, which allows the vascular status to be analysed in depth. The ratio of LA to the circumscribed total choroidal area (TCA) gives the CVI value. In the literature, the choroidal thickness and choroidal vessel diameter, markers that represent the choroidal vascular status, have been explored in patients with PXG [21,22,23,24,25]. However, the CVI of patients with PXG has not been investigated before. Our hypothesis was that there are choroidal vascular alterations in both eyes of asymmetric PXG patients. Thus, the present study explored the CVI of the macula (mCVI) and peripapillary (pCVI) regions in the affected eyes and non-glaucomatous fellow eyes without clinically PXM of the patients with asymmetric PXG and compared the results with those of healthy control participants.
Materials and methods
Study participants
This cross-sectional, and comparative study evaluated patients with asymmetric PXG and normal eyes in our tertiary eye care centre. Our research was approved by the Institutional Ethics Review Board of Ankara Training and Research Hospital, Turkey, and conducted in agreement with the tenets of the Declaration of Helsinki. The study was carried out in 10 months between February and November 2020. All of the participants provided written informed consent for all procedures.
The participants were divided into three groups: (1) the affected eyes of patients with asymmetric PXG (glaucomatous eyes), (2) clinically PXM-negative, non-glaucomatous contralateral eyes of these patients (fellow eyes), and (3) only the right eyes of healthy participants without any other ocular diseases (control eyes).
The examinations, inclusion and exclusion criteria
Patients with PXG were diagnosed based on the combined existence of clinically PXM and glaucomatous damage according to the European Glaucoma Association Guidelines [26]. PXM was detected on the surface of the anterior lens capsule and/or at the pupillary border and/or on the trabecular meshwork with gonioscopy in the slit-lamp biomicroscopy. In addition, the loss of a pupillary ruff and the presence of iris transillumination defects also supported the diagnosis of PXM. Glaucoma was diagnosed in the presence of a combination of findings regarding the optic nerve head’s appearance on fundoscopy, the IOP value obtained with Goldmann applanation tonometry (GAT), a standard automated perimetry analysis (Swedish Interactive Threshold Algorithm 24-2 test of the Humphrey Visual Field [VF] analyser 750i; Carl Zeiss Meditec Inc., Dublin, CA, USA), and the retinal nerve fibre layer (RNFL) measurement obtained via OCT. All of the glaucomatous eyes in the present study consisted of patients whose condition was under control using topical anti-glaucomatous medication. The contralateral fellow eyes of these patients had the following characteristics: clinically PXM-negative, no glaucomatous optic nerve head morphology, no VF damage, no RNFL loss on OCT, and repeated IOP measurements <21 mmHg. The diagnoses and follow-up examinations of the patients with asymmetric PXG were conducted by the same experienced glaucoma specialist (U.E.).
Patients with the following situations were not included in the present research: another type of glaucoma other than PXG; clinically bilateral involvement of PXM; a history of ocular or orbital trauma; any past ocular surgery or laser treatment; a history of ischaemic or non-ischaemic optic neuropathies; optic disc anomalies, including coloboma, optic pit, tilted disc, and optic disc drusen; any retinal vascular diseases, including diabetic retinopathy, hypertensive retinopathy, and vascular occlusion; a spherical and/or cylindrical refractive error >3 dioptres (D); an axial length (AL) > 24 mm and <22 mm; any haematological or immunological diseases; uncontrolled hypertension that was currently receiving systemic medical treatment; smokers; and current or past drug and alcohol abuse that might have caused impairments in vascular perfusion.
All of the participants underwent a thorough ophthalmologic examination, including an anterior and a dilated posterior segment examination using a slit-lamp biomicroscope. The participants’ demographic data, best-corrected visual acuity (BCVA) as measured by a Snellen chart (as converted LogMAR equivalent), gonioscopy via a Goldmann three-mirror lens, and IOP values assessed using a GAT were recorded. The cup-to-disc ratio was evaluated as vertical. The spherical equivalent (SE) was calculated as the sum of the full spherical power and half of the cylindrical power. The mean arterial pressure (MAP) was calculated as follows: diastolic blood pressure + 1/3 (systolic blood pressure − diastolic blood pressure). The central corneal thickness (CCT) and AL were measured using a Lenstar LS 900 (Hagg-Streit AG, Koeniz, Switzerland).
OCT imaging
After a complete biomicroscopic examination was conducted, RNFL analysis mode for the optic nerve head and the enhanced depth imaging mode examinations for the macula were carried out using spectral-domain optical coherence tomography (SD-OCT; Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany) by the same experienced medical technician. All OCT measurements were performed during the same time period (between 09:00 and 11:00 a.m.) to avoid diurnal fluctuations. Furthermore, all scans were acquired following pupil dilation. Macular OCT images were procured using a horizontal scan centred on the central foveal region (Fig. 1A) and peripapillary OCT images were achieved using a 3.4-mm-diameter, 360-degree-circle scan centred on the optic nerve head (Fig. 2A). Only high-quality scans (≥25 Q) were included in the data analysis. The images were viewed and measured using Heidelberg Eye Explorer software (Heidelberg Eye Explorer version 1.8.6.0; Heidelberg Engineering). The obtained raw OCT data (macula and peripapillary) were evaluated with an image-processing programme for advanced analysis.
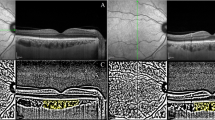
A Illustration of macular CVI measurement using ImageJ software. The binarized image to view the choroid-scleral junction using the auto local threshold tool (Niblack Method, 8-bit type) (B), A grey reference line of 1500 µm in length using the line tool and circumscribed submacular choroidal area using the polygon tool (C), yellow lines representing the LA (dark pixels) using the colour threshold tool (Red-Green-Blue colour type) (D).
A Illustration of peripapillary CVI measurement using ImageJ software. The segmented peripapillary images (T temporal, ST superior-temporal, SN superior-nasal, N nasal, IN inferior-nasal, and IT inferior-temporal) (B), the binarized peripapillary image enabling the evaluation of all sectors separately (C).
Image acquisition and processing
Image processing was carried out with public domain software (http://fiji.sc/). It was binarized using the protocol described by Agrawal et al. [27]. Briefly, the raw OCT scans were opened using the ImageJ programme (version 1.53a; National Institutes of Health, Bethesda, MD, USA). First, the scale was adjusted for a unit of length (as µm) and pixel distance (as 200 µm). The image was converted to an 8-bit type and was binarized to view the choroid-scleral junction using the autolocal threshold tool (Niblack Method, Fig. 1B). A grey reference line of 1500 µm in length was drawn parallel to the retinal pigment epithelium using the line tool. The TCA was selected as the region between the retinal pigment epithelium and the choroid-scleral junction using the polygon tool (Fig. 1C). This region was added to the region of interest (ROI) manager. Next, the colour threshold tool and Red-Green-Blue colour type were used to select the dark pixels expressing the LA. The LA region was saved in the ROI manager (Fig. 1D). Both areas in the ROI manager were selected and merged via the “AND” command.
In the macula, a 750-µm width was provided on two sides from the fovea. In the peripapillary area, scans were segmented using the methods described by Pellegrini et al. [28] and Park et al. [29] (Fig. 2B). Accordingly, in these scans, 45° for the superior-temporal (ST), superior-nasal (SN), inferior-temporal (IT), and inferior-nasal (IN) sectors and 90° for the nasal (N) and temporal (T) sectors of the peripapillary area were binarized separately (Fig. 2C).
The CVI was calculated as the ratio of LA to TCA. The SA was indicated by light-coloured pixels and was calculated by subtracting the LA from the TCA. All these measurements were obtained separately by two experienced observers (M.S. and O.I.) who were blinded to the participants’ clinical information. The means of both values were included in the analysis. Furthermore, the interobserver and intraobserver reliability of the mCVI and the pCVI calculations were determined using intraclass correlation coefficients (ICC) with a 95% confidence interval (CI). The ICC value was acceptable when its range fell between 0.75 and 0.90 and was excellent when its value was greater than 0.90 [30].
Statistical analysis
The results of a priori power analysis, using power analysis and sample size (PASS, version 13; NCSS, LLC, Kaysville, UT) calculation software, required at least 44 eyes per group for a power of 90%.
Statistical analyses were performed using the statistical package for the social sciences (SPSS) software (SPSS Inc., version 22; Chicago, IL, USA). The normality of data was tested using both visual (histograms and probability plots) and analytical (Kolmogorov–Smirnov/Shapiro–Wilk test) methods. The descriptive analysis was presented using the mean and standard deviation. The Levene test was employed to check the homogeneity of the variances. A Chi-square test was used to analyse the categorical variables. The pairwise comparison tests (independent samples t-test and paired samples t-test) were performed between the groups. A Bonferroni correction was applied for multiple pairwise comparisons.
Finally, univariate and multiple linear backward regression analyses were carried out to identify the effects of ocular-systemic factors (independent variables) on the mCVI and pCVI (dependent variables). The factors that showed significant associations in the univariate analysis (p < 0.05) were included in the multiple linear regression model. In the regression tests, a standardised beta coefficient (ß) was presented. A p-value of <0.05 was considered statistically significant.
Results
The present study enrolled a total of 144 eyes, including both eyes of 48 asymmetric PXG patients and the right eyes of 48 control individuals. There was no significant difference between the glaucoma patients and the control participants with respect to age (57.6 ± 4.5 years vs. 57.1 ± 3.4 years, respectively; p = 0.891) and sex (28 females vs. 26 females, respectively; p = 0.710). There were also no significant differences among the groups in terms of SE, CCT, MAP, and AL (all p > 0.05). The glaucomatous eyes had significantly higher IOP and cup-to-disc ratio values than the fellow eyes (p = 0.001 and p < 0.001) and the control eyes (p = 0.001 and p < 0.001). There were no significant differences between the fellow and control eyes in terms of the IOP and cup-to-disc ratio (p > 0.05 for both). The visual field MD and PSD values were significantly higher in the glaucomatous eyes compared with the fellow eyes (p < 0.001 and p = 0.005) and the control eyes (p < 0.001 for both). The glaucomatous eyes demonstrated RNFL thinning in all sectors compared to the control eyes (all p < 0.01). There was no difference in RNFL thicknesses and visual field indices between the fellow eyes and the control eyes (all p > 0.05; Table 1).
The ICC value obtained in our research demonstrated an excellent reliability agreement with an interobserver range from 0.917 to 0.961 and an intraobserver range from 0.958 to 0.986 (Supplementary Table 1).
The choroidal vascular measurements of the macula and optic nerve head are presented in Table 2. In the glaucomatous eyes, all of the peripapillary sectors and macula had decreased CVI values compared with the contralateral eyes and control individuals (all p < 0.01). When compared with the control eyes, the fellow eyes showed lower CVI scores in all peripapillary sectors (all p < 0.01) except for the nasal and superior-nasal sectors. Furthermore, the fellow eyes had a lower value in the mCVI than the control eyes (p = 0.015).
Table 3 shows the regression analysis between the CVI and the ocular-systemic variables. Univariate regression analysis had a significant effect on the CVI for four parameters (IOP, MAP, cup-to-disc ratio, and AL; all p < 0.05) in the glaucomatous and fellow eyes. In the multivariate regression model, the cup-to-disc ratio was negatively associated with the mCVI (ß = −0.185, p = 0.035) and pCVI (ß = −0.370, p < 0.001) in the glaucomatous eyes. In the fellow eyes, the cup-to-disc ratio was only associated with pCVI (ß = −0.263, p = 0.007) but not with mCVI (p = 0.087). The AL showed a significantly negative correlation with mCVI in the glaucomatous eyes (ß = −0.268, p = 0.010) and in the fellow eyes (ß = −0.192, p = 0.033).
Discussion
Glaucoma is a sight-threatening disease with characteristic optic nerve damage. Functional visual loss can be minimised with an early diagnosis and timely interventions. Identification of structural defects is important for the visual prognosis before functional damage develops. With recent advances in imaging methods, structural defects or changes can often be detected much earlier [31]. The present research used an OCT-based image binarization technique to assess the macular and peripapillary choroidal vascularity in patients with asymmetric PXG. The glaucomatous eyes had significantly lower mCVI and pCVI scores in all sectors than the contralateral fellow eyes and the control eyes. Furthermore, non-glaucomatous contralateral eyes without PXM had significantly lower mCVI and pCVI scores in all sectors, except for the SN and N sectors, than the control eyes. Decreased CVI in glaucomatous eyes and fellow eyes may result in inadequate retinal and optic nerve nutrition. Thus, it may play a role in the glaucomatous ischaemic injury. In addition, the subclinical choroidal ischaemic process may have started in the fellow eyes without clinically apparent PXM.
When compared to primary open-angle glaucoma (POAG), PXG is characterised by a more aggressive clinical course, a higher degree of IOP and more severe optic nerve damage at the time of diagnosis, a faster disease progression, a poor response to medical treatment, and an increased need for surgical intervention [32, 33]. Park and colleagues reported that eyes with PXG had reduced average peripapillary vessel density in the radial peripapillary capillaries compared to eyes with POAG matched for glaucoma severity [34]. Similarly, Rebolleda et al. found decreased peripapillary capillary density in PXG than in POAG at similar glaucoma damage [35]. As a result of these studies, it was concluded that vascular impairment might be an additional risk factor for the rapid deterioration and aggressive nature observed in eyes with PXG.
PXM deposits accumulate not only intraocularly but also in many organs/systems, including vessels. The presence of PXM in vascular endothelial cells, smooth muscle cells, and pericytes has been demonstrated [36]. Previous studies found PXM to be associated with circulation impairment and ischaemic changes in several organs, especially in the cardiac and cerebral systems, as a result of vascular involvement [12]. Helbig et al. identified PXM fibrils in the iris vessel from the adventitia to the endothelium using electron microscopy [14]. They concluded that a gradual degeneration of iris vascular cells induced anterior chamber hypoxia. In another study by Parodi and colleagues, iris angiography revealed hypoperfusion, microneovascularization, and anastomotic vessels [15]. In an electron microscopy study, PXM was also identified within the walls of the posterior ciliary arteries, central retinal artery, and vortex veins in both affected and unaffected fellow eyes of patients with clinically unilateral PXS [12]. In subsequent clinical studies, it was demonstrated that the ophthalmic artery and retrobulbar vessels had decreased blood flow velocities and increased resistive indices using the colour Doppler imaging method [13, 14]. In our study, we obtained findings compatible with the literature using a new imaging method. Therefore, our results are important and may guide studies on the effect of vascular pathways in the pathogenesis of PXG.
The effects of pseudoexfoliation on quantitative choroidal biomarkers have been comprehensively examined. The most well-known of these biomarkers is the choroidal thickness (CT) measurement. Varying results associated with the CT have been reported in previous studies. Bayhan and colleagues detected thinning only in the nasal choroid in PXG patients compared to control participants (182.12 ± 39.88 vs. 201.56 ± 32.00 at 1.5 mm and 126.47 ± 32.12 vs. 146.60 ± 31.37 at 3.0 mm, respectively) [22]. Egrilmez et al. identified thinning in the nasal, subfoveal, and temporal choroid (203.70 ± 27.48 vs. 221.03 ± 31.95 at 1.5 mm, 241.43 ± 32.47 vs. 268.03 ± 24.50, and 222.03 ± 27.78 vs. 238.40 ± 20.78 at 1.5 mm, respectively) compared to controls [37]. Dursun and colleagues observed a thinner CT in PXG patients not only in the nasal-to-temporal macula but also in the peripapillary area in all sectors when compared with healthy participants [23]. Moghimi et al. concluded that the severity of PXG was significantly associated with a decrease in peripapillary CT [24]. In contrast, Ozge and colleagues found no differences in both the macula and peripapillary CT measurements among PXG and PXS patients and control cases [38]. According to the findings in the literature, the CT tends to decrease in PXG patients, while the results in PXS patients are conflicting. Therefore, the most important difference in our study was that there was no clinically PXM identified in the contralateral eyes. Our findings reflected the probable preclinical effects of PXM in fellow eyes compared to control eyes.
In the study by Suh et al., peripapillary choroidal thinning was not associated with impaired choroidal perfusion in glaucomatous eyes [39]. Since the choroid consists of both vascular and connective tissue, the CT might provide information indirectly about choroidal vascularity. In contrast, the CVI allows a separate evaluation of the vascular area in the choroid regardless of the SA. While the CT evaluates the distance between the retinal pigment epithelium and the choroid-scleral junction only at certain points, the CVI may ensure more accurate information about the percentage of the choroidal LA in the entire selected area. Additionally, the CT is associated with most patient variables, such as age, refractive error, AL, IOP, systolic blood pressure, and diurnal variation [20]. The CVI is considered to be a more robust indicator and also a promising marker for choroidal vascularity as it is not affected by various physiological factors.
There were some limitations in this research. First, the cross-sectional method used in our study cannot provide precise data on the disease progression of these eyes or the presence of any possible vascular pathogenesis. A clearer view of the vascular pathway in the glaucoma process can be achieved through cohort studies and the participation of multiple centres. However, our research is the first on CVI in non-PXM fellow eyes in the literature and will increase interest in the vascular pathway for future pseudoexfoliation studies. The second limiting factor of our research, as in other similar studies, was that some technical limitations could cause CVI variations [20]. Briefly, these included a lower quality of scans, shadowing in the choroid due to larger retinal vascular patterns, a deficiency in spatial resolution affecting the easy differentiation of the luminal and stromal components of the choriocapillaris with current imaging technology, and the use of a manual measurement-based system. However, only high-quality scans were involved in our research to minimise variations in the CVI. The high ICC value we obtained was also another strength of our work.
In conclusion, our study demonstrated a reduction in the macular and peripapillary choroidal vascularity in the affected eyes and PXM-negative contralateral eyes of patients with asymmetric PXG when compared with the healthy control participants. These findings suggest that the CVI may serve as a useful marker and an adjunctive structural diagnostic tool. Prospective multi-centre investigations may provide better insight into the changes of choroidal vascularity and its relationship to the pathogenesis of glaucoma.
Summary
What was known before
-
The structure–function relationship in the early stages of glaucoma is complexly associated. Vascular dysfunction and ischaemic process play a role in the vascular theory of the development of glaucoma.
What this study adds
-
Reduced macular and peripapillary choroidal vascularity index were detected in the non-glaucomatous contralateral fellow eyes without clinical pseudoexfoliation of pseudoexfoliative glaucoma patients when compared to controls. This finding may indicate vascular involvement that predisposes to early structural damage.
References
Chauhan BC. Detection of glaucoma: the role of new functional and structural tests. Curr Opin Ophthalmol. 2004;15:93–5.
Grieshaber MC, Mozaffarieh M, Flammer J. What is the link between vascular dysregulation and glaucoma? Surv Ophthalmol. 2007;52:S144–154.
Wareham LK, Calkins DJ. The neurovascular unit in glaucomatous neurodegeneration. Front Cell Dev Biol. 2020;8:452.
Cherecheanu AP, Garhofer G, Schmidl D, Werkmeister R, Schmetterer L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr Opin Pharmacol. 2013;13:36–42.
Schlötzer-Schrehardt U, Zenkel M. The role of lysyl oxidase-like 1 (LOXL1) in exfoliation syndrome and glaucoma. Exp Eye Res. 2019;189:107818.
Elhawy E, Kamthan G, Dong CQ, Danias J. Pseudoexfoliation syndrome, a systemic disorder with ocular manifestations. Hum Genom. 2012;6:22.
Holló G. Vascular dysfunction in exfoliation syndrome. J Glaucom. 2018;27:S72–S74.
Parekh P, Green WR, Stark WJ, Akpek EK. Electron microscopic investigation of the lens capsule and conjunctival tissues in individuals with clinically unilateral pseudoexfoliation syndrome. Ophthalmology. 2008;115:614–9.e2.
Zheng X, Sakai H, Goto T, Namiguchi K, Mizoue S, Shiraishi A, et al. Anterior segment optical coherence tomography analysis of clinically unilateral pseudoexfoliation syndrome: evidence of bilateral involvement and morphologic factors related to asymmetry. Investig Ophthalmol Vis Sci. 2011;52:5679–84.
Arnarsson A, Sasaki H, Jonasson F. Twelve-year incidence of exfoliation syndrome in the Reykjavik eye study. Acta Ophthalmol. 2013;91:157–62.
Puska PM. Unilateral exfoliation syndrome: conversion to bilateral exfoliation and to glaucoma: a prospective 10-year follow-up study. J Glaucom. 2002;11:517–24.
Schlötzer-Schrehardt U, Küchle M, Naumann GO. Electron-microscopic identification of pseudoexfoliation material in extrabulbar tissue. Arch Ophthalmol. 1991;109:565–70.
Yüksel N, Karabaş VL, Arslan A, Demirci A, Cağlar Y. Ocular hemodynamics in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Ophthalmology. 2001;108:1043–9.
Dayanir V, Topaloğlu A, Ozsunar Y, Keceli M, Okyay P, Harris A. Orbital blood flow parameters in unilateral pseudoexfoliation syndrome. Int Ophthalmol. 2009;29:27–32.
Çınar E, Yüce B, Aslan F. Retinal and choroidal vascular changes in eyes with pseudoexfoliation syndrome: a comparative study using optical coherence tomography angiography. Balk Med J. 2019;37:9–14.
Li F, Ma L, Geng Y, Yan X, Zhang H, Tang G, et al. Comparison of macular choroidal thickness and volume between pseudoexfoliative glaucoma and pseudoexfoliative syndrome. J Ophthalmol. 2020;2020:8886398.
Goharian I, Sehi M. Is there any role for the choroid in glaucoma? J Glaucom. 2016;25:452–8.
Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol. 1969;53:721–48.
Iovino C, Pellegrini M, Bernabei F, Borrelli E, Sacconi R, Govetto A, et al. Choroidal vascularity index: an in-depth analysis of this novel optical coherence tomography parameter. J Clin Med. 2020;9:595.
Agrawal R, Ding J, Sen P, Rousselot A, Chan A, Nivison-Smith L, et al. Exploring choroidal angioarchitecture in health and disease using choroidal vascularity index. Prog Retin Eye Res. 2020;77:100829.
Demircan S, Yılmaz U, Küçük E, Ulusoy MD, Ataş M, Gülhan A, et al. The effect of pseudoexfoliation syndrome on the retinal nerve fiber layer and choroid thickness. Semin Ophthalmol. 2017;32:341–7.
Bayhan HA, Aslan Bayhan S, Can I. Evaluation of the macular choroidal thickness using spectral optical coherence tomography in pseudoexfoliation glaucoma. J Glaucom. 2016;25:184–7.
Dursun A, Ozec AV, Dogan O, Dursun FG, Toker MI, Topalkara A, et al. Evaluation of choroidal thickness in patients with pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J Ophthalmol. 2016;2016:3545180.
Moghimi S, Nekoozadeh S, Motamed-Gorji N, Chen R, Fard MA, Mohammadi M, et al. Lamina cribrosa and choroid features and their relationship to stage of pseudoexfoliation glaucoma. Investig Ophthalmol Vis Sci 2018;59:5355–65.
Sarrafpour S, Adhi M, Zhang JY, Duker JS, Krishnan C. Choroidal vessel diameters in pseudoexfoliation and pseudoexfoliation glaucoma analyzed using spectral-domain optical coherence tomography. J Glaucom. 2017;26:383–9.
European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition—Chapter 2: classification and terminology supported by the EGS foundation: part 1: foreword; introduction; glossary; chapter 2 classification and terminology. Br J Ophthalmol. 2017;101:73–127.
Agrawal R, Salman M, Tan KA, Karampelas M, Sim DA, Keane PA, et al. Choroidal vascularity index (CVI)—a novel optical coherence tomography parameter for monitoring patients with panuveitis? PLoS ONE. 2016;11:e0146344.
Pellegrini M, Giannaccare G, Bernabei F, Moscardelli F, Schiavi C, Campos EC. Choroidal vascular changes in arteritic and nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2019;205:43–9.
Park JW, Suh MH, Agrawal R, Khandelwal N. Peripapillary choroidal vascularity index in glaucoma—a comparison between spectral-domain OCT and OCT angiography. Invest Ophthalmol Vis Sci. 2018;59:3694–701.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63.
Rodriguez-Una I, Azuara-Blanco A. New technologies for glaucoma detection. Asia Pac J Ophthalmol. 2018;7:394–404.
Desai MA, Lee RK. The medical and surgical management of pseudoexfoliation glaucoma. Int Ophthalmol Clin. 2008;48:95–113.
Konstas AG, Hollo G, Astakhov YS, Teus MA, Akopov EL, Jenkins JN, et al. Factors associated with long-term progression or stability in exfoliation glaucoma. Arch Ophthalmol. 2004;122:29–33.
Park JH, Yoo C, Girard MJA, Mari JM, Kim YY. Peripapillary vessel density in glaucomatous eyes: comparison between pseudoexfoliation glaucoma and primary open-angle glaucoma. J Glaucoma. 2018;27:1009–16.
Rebolleda G, Pérez-Sarriegui A, De Juan V, Ortiz-Toquero S, Muñoz-Negrete FJ. A comparison of two optical coherence tomography-angiography devices in pseudoexfoliation glaucoma versus primary open-angle glaucoma and healthy subjects. Eur J Ophthalmol. 2019;29:636–44.
Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45:265–315.
Egrilmez ED, Karadeniz Ugurlu S, Sahin Atik S, Guven YZ. The effect of pseudoexfoliation syndrome on choroidal thickness in open-angle glaucoma. Arq Bras Oftalmol. 2019;82:400–6.
Ozge G, Koylu MT, Mumcuoglu T, Gundogan FC, Ozgonul C, Ayyildiz O, et al. Evaluation of retinal nerve fiber layer thickness and choroidal thickness in pseudoexfoliative glaucoma and pseudoexfoliative syndrome. Postgrad Med. 2016;128:444–8.
Suh MH, Park JW, Khandelwal N, Agrawal R. Peripapillary choroidal vascularity ındex and microstructure of parapapillary atrophy. Investig Ophthalmol Vis Sci. 2019;60:3768–75.
Funding
The authors indicate they have no financial disclosures. The authors, their families, their employers and their business associates have no financial or proprietary interest in any product or company associated with any device, instrument or drug mentioned in this article. The authors have not received any payment as consultants, reviewers or evaluators of any of the devices, instruments or drugs mentioned in this article.
Author information
Authors and Affiliations
Contributions
MS was responsible for designing the study conceptualisation, conducting the search, applying the ethical committee, screening potentially relevant studies, collecting the data and patient’s information, extracting and analysing data, interpreting results, writing the draft. OI was responsible for designing the study conceptualisation, conducting the search, screening potentially relevant studies, collecting the data and patient’s information, extracting and analysing data. ES was responsible for performing statistical analysis and supervision, providing potentially eligible participants, correcting and revising the final version of the manuscript. UE was responsible for performing supervision and feedback, providing potentially eligible participants, correcting and revising the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ankara Education and Research Hospital and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Disclosure statement
The authors indicate no financial support, budget, or payment. This article has been read and approved by all the authors. The authors indicate no financial support, budget, grant, or payment.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Simsek, M., Inam, O., Sen, E. et al. Analysis of the choroidal vascularity in asymmetric pseudoexfoliative glaucoma using optical coherence tomography-based image binarization. Eye 36, 1615–1622 (2022). https://doi.org/10.1038/s41433-021-01700-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01700-0
This article is cited by
-
Choroidal vascularity index in pseudoexfoliation syndrome: a review of the literature
Spektrum der Augenheilkunde (2023)