Abstract
Neuro-ophthalmic features are a known association in tuberculosis, especially common in central nervous system tuberculosis (CNS-TB). They are mostly the result of the visual pathway and/or ocular motor and other cranial nerve involvement. Furthermore, toxic optic neuropathy and paradoxical response to anti-tubercular drugs (ATT) are also not uncommon. The etiopathogenesis is by the complex interplay of various factors like exudates, vasculitis, arachnoiditis, presence of tuberculomas, hydrocephalus, brain infarcts and/or immune-mediated reaction. The entity often poses a diagnostic dilemma for the ophthalmologists/neuro-ophthalmologists and may lead to irreversible vision loss. The presence of neuro-ophthalmic features not only affect the visual outcome but are also predictors of systemic morbidity of the disease. Therefore, understanding and knowledge about this entity are necessary for the comprehensive management of the disease. While various forms of TB including CNS-TB have been well-dealt with in literature, little is discussed specifically about the neuro-ophthalmic manifestations of tuberculosis. Therefore, the purpose of this review is to highlight current understanding of the types of neuro-ophthalmic involvement in tuberculosis, its etiopathogenesis, diagnosis and management.
摘要
摘要
众所周知结核病与神经眼科特征有关, 尤其是在中枢神经系统结核病 (central nervous system tuberculosis, CNS-TB) 常见。它们大多是视觉通路和/或眼运动和其他颅神经受累的结果。此外, 毒性视神经病变和抗结核药物 (anti-tubercular drugs, ATT) 的副作用也并不少见。其病因是由于各种因素如渗出、血管炎、蛛网膜炎、结核瘤、脑积水、脑梗塞和/或免疫因素介导的复杂的相互作用引起的。这种复杂的相互作用网络给眼科医生/神经眼科医生对于疾病诊断的困境, 并可导致不可逆的视力丧失。神经眼科特征的存在不仅影响视力结局, 也是疾病系统发病率的预测因素。因此, 了解和学习这个知识体系对于疾病的综合管理是必要的。虽然文献中对包括CNS-TB在内的各种类型的结核都有很好的论述, 但很少有人针对结核的神经眼科表现进行讨论。因此, 本综述旨在强调结核合并神经眼科疾病的类型、发病机制、诊断和治疗。
Similar content being viewed by others
Introduction
Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis, is one of the major causes of morbidity and mortality worldwide. As per the World Health Organization (WHO), around ten million people were affected by TB globally and nearly 1.5 million died in 2018 alone. Countries from South East Asia region account for the maximum number of cases with India leading the count [1]. The emergence of the human immunodeficiency virus (HIV) co-infection [2] and drug-resistant forms of TB have further contributed to the disease morbidity.
Although TB can affect any part of the body, central nervous system (CNS) infection is one of the most devastating forms of the disease accounting for 5–10% of cases of extrapulmonary TB [3, 4]. Central nervous system tuberculosis (CNS-TB) manifests primarily as meningitis [5], focal intraparenchymal lesions like tuberculomas, spinal arachnoiditis and rarely as encephalitis and brain abscess. Neuro-ophthalmic manifestations of the disease, which are mostly the result of the visual pathway and/or ocular motor and other cranial nerve involvement, may not be uncommon in CNS-TB. Furthermore, toxic optic neuropathy and paradoxical response to anti-tubercular drugs (ATT) may be seen in any form of TB. The entity often poses a diagnostic dilemma for the ophthalmologists and is associated with significant visual as well as systemic morbidity [6, 7].
While various forms of TB including CNS-TB have been well-dealt with in literature, little is discussed specifically about the neuro-ophthalmic manifestations of the disease. Therefore, the purpose of this review is to highlight the current understanding of types of neuro-ophthalmic involvement in TB, its etiopathogenesis, diagnosis and management. The search of published literature for this review article was done using Pubmed, Medline, Embase and Ovid extending over the last five decades along with checking for various cross-references. The articles available in the English language with full-text access were included and an electronic literature search was performed using keywords like TB, neuro-ophthalmic features in TB, CNS-TB, tuberculomas, tuberculous optic neuropathy, toxic optic neuropathy, paradoxical response to anti-tubercular drugs, cranial nerve palsy, vision loss, diagnosis and management in TB.
Etiopathogenesis
As the neuro-ophthalmic features of TB are mostly part of the CNS disease, the etiopathogenesis of the two essentially remains the same. CNS acquires infection when the bacilli reach the brain and meninges as a result of bacteraemia (during primary infection) and form multiple small tubercles, called the ‘Rich foci’. Juxtaependymal or subependymal location of these tubercles favour the development of tuberculous meningitis (TBM), which is the most common form of CNS-TB. It is characterized by chronic basilar meningitis with meningeal inflammation, gelatinous exudates, intracranial vasculitis and obstructive or communicating hydrocephalus. Vasculitis in TBM involves both arteries and veins [8]. Obstruction to cerebrospinal fluid (CSF) flow is caused by the thick exudates in the aqueduct or the fourth ventricle. The disease has a predilection for the basal parts of the brain, which lie in close proximity with the anterior visual pathway, thus commonly presenting with vision loss.
Tuberculomas that develop by the enlargement of brain tubercles are another form of CNS-TB that may be seen alone or along with TBM. It is characterised by a granulomatous reaction comprising of Langerhan’s cells, epitheloid cells and lymphocytes with a central core of caseous material and viable tubercle bacilli. The appearance of the tuberculomas may vary clinically. Some may resemble tuberculous abscess, meningioma etc. and rarely show cystic changes [9]. Upregulation of tumour necrosis factor (TNF-α) from the microglial cells and matrix metalloproteinases (MMP) from the monocytes, especially MMP-9 facilitate tuberculoma formation. MMP-9 is involved in macrophage recruitment and TNF-α causes macrophage activation, cytokine production and apoptosis. While tuberculoma formation is considered as a part of the host defence mechanism to contain the infection, it also shields the viable bacteria from the immune-mediated response [10, 11].
Tubercular brain abscess is a rare manifestation that may occur either by the spread of infection from meninges or the tubercular granulomas. But unlike tubercular granulomas, tuberculous abscess does not exhibit granulomatous reaction and mainly contains pus with abundant live organisms [12].
Several factors contribute to vision loss and other neuro-ophthalmic features in TBM. Exudates especially in the interpeduncular, suprasellar and Sylvian cisterns directly damage the encasing structures like optic chiasma and cranial nerve roots arising from the ventral aspect of the brain. Associated optochiasmatic arachnoiditis, vasculitis, hydrocephalus and brain infarcts also add to the insult. Rarely, a paradoxical reaction may occur during the course of anti-tubercular treatment (ATT) administered for CNS or pulmonary TB leading to optochiasmatic arachnoiditis or development of new tuberculomas and/or expansion of the existing ones in this region [13,14,15]. This is attributed to the exaggerated host reaction against the tuberculous protein released by the dying bacteria [16] Optochiasmatic arachnoiditis either causes mechanical compression of the intracranial part of the anterior visual pathway or results in vasculitis and interruption of the blood supply, leading to severe vision loss [10]. Tuberculomas may exhibit features similar to any space-occupying lesion with compression of key structures. It may involve the pituitary, sella, occipital lobe, optic disc and brain stem.
One must note that specific cohorts have shown genetic susceptibility to tuberculous infection related to variations in genes encoding for the recognition receptors. Toll-like receptor TLR2 genotype 597CC has been linked with disseminated forms of TB (miliary and meningitis) caused by a specific strain of mycobacterium called the ‘Beijing genotype’ in Vietnam. Likewise, Arg753Gln and Arg677Trp have been associated with increased susceptibility to TB in Turkish and Tunisian cohorts, respectively [17].
Clinical features
The occurrence of neuro-ophthalmic complications is a common association in TB, especially CNS-TB, reported in nearly 82% cases of TBM [18] and 67% cases of paediatric tuberculous meningoencephalitis [6]. Table 1 summarizes the various neuro-ophthalmic manifestations of the disease.
Vision loss
There can be a myriad of ocular signs and symptoms but the most common manifestation is vision impairment described in as high as 72% of cases of TBM [19].
Tuberculous optic neuropathy
Tuberculous optic neuropathy is an important cause of vision loss that may occur from infectious infiltration of the nerve or inflammation by basal exudates and endarteritis of the supplying vasculature causing ischaemia. The infectious infiltration may occur either through contiguous spread from adjacent structures like choroid plexus or by haematogenous dissemination from a distant source like lungs. Sometimes, optic neuropathy can occur due to associated hypersensitivity reaction or toxicity to anti-tubercular drugs.
Optic nerve affliction may be unilateral or bilateral and can be in the form of optic neuritis with disc swelling, i.e. papillitis or neuroretinitis, or without disc swelling, i.e. retrobulbar neuritis or optic perineuritis. Optic disc swelling may also be associated with tuberculous posterior scleritis. Rarely, there can be optic nerve tubercles [20]. Papillitis (51.6%) was the most common presentation noted in a retrospective study defining the clinical spectrum of tuberculous optic neuropathy. Other less common manifestations in this study were neuroretinitis (14.5%), optic nerve tubercles (11.3%), retrobulbar neuritis (8.1%) and optic neuritis (8.1%) [21]. Majority of the patients in this study presented with painless loss of vision and some with ocular pain, visual field defects, colour vision defects, diplopia and ptosis [21]. In a series of paediatric meningoencephalitis cases, retrobulbar neuritis (32%) was found to be the most frequent neuro-ophthalmic finding [6]. Other forms of optic nerve involvement described are compressive optic neuropathy, anterior ischaemic optic neuropathy, orbital apex syndrome and rarely central retinal vein occlusion or posterior scleritis [21]. Clinically patients present with mostly unilateral and sometimes bilateral visual loss that may range from mild reduction in visual acuity to absent perception of light progressing over a few weeks. Pain, relative afferent pupillary defect (RAPD), dyschromatopsia and visual field defects are other important features. Dyschromatopsia is often out of proportion to vision loss [22]. The common visual field defects are central scotomas and enlargement of the blind spot. Periocular pain occurs in the majority of patients with optic perineuritis.
Optic nerve perineuritis, characterized by the inflammation of optic nerve sheath and adjacent tissue, is a rare association of TB [23, 24]. It may be the result of infection, ATT toxicity or rarely dysimmune neuropathy [23]. Vision loss can be attributed to the thickened optic nerve sheath that leads to circumferential compression of the nerve, resultant vascular occlusion and infarction. Optic nerve perineuritis can often be confused with optic neuritis due to overlapping clinical features. However, older age of onset (>45 years), pain out of proportion to vision loss, sparing of central vision and progression of vision loss for >2 weeks usually go in favour of perineuritis. Radiological findings can further confirm the diagnosis. Recurrences are common in optic perineuritis especially on discontinuation of steroids. Unlike in optic neuritis (where corticosteroids do not alter the final visual outcome), patients with optic perineuritis show a dramatic response to steroids and if left untreated can lose vision. Therefore, prolonged therapy with higher dose of oral steroids (prednisone, 80 mg/day) is indicated in optic perineuritis [25]. Neuroretinitis, although rarely seen in TB, needs to be differentiated from other causes like cat scratch disease (CSD) and recurrent idiopathic neuroretinitis. CSD should be suspected in patients with a history of cat exposure, associated systemic symptoms and poor visual acuity despite small or no RAPD. Recurrent idiopathic neuroretinitis has a recurrent course and more extensive optic nerve enhancement on MRI [26].
Choroid tubercles may be seen in miliary TB that may represent the immune-mediated hypersensitivity reaction to the tubercular antigens [27].
Raised intracranial pressure
Raised intracranial pressure [6], hydrocephalus and papilloedema [7, 28] are other notable associations in TBM leading to vision loss that occurs by a complex interplay of the above factors. Dilatation of the third ventricle in obstructive hydrocephalus may lead to optic chiasmal compression [29]. It may also cause raised intracranial pressure that along with other factors like cerebral oedema, tuberculomas and exudates interferes with axoplasmic flow resulting in papilloedema and consequent secondary optic atrophy [30]. In fact, the presence of hydrocephalus is an indicator of greater disease severity and mortality [31].
Optochiasmatic arachnoiditis and tuberculoma/paradoxical response to ATT
Optochiasmatic arachnoiditis and optochiasmatic tuberculoma are also important causes of vision impairment in TB. It may be the result of the infectious process but usually is seen as a paradoxical reaction to ATT. The paradoxical response is a delayed type of hypersensitivity reaction occurring at around 4–8 weeks after initiation of ATT [16, 32]. Vision loss is usually severe, insidious (sometimes acute) and progressive with impaired pupillary reactions, hemianopic or concentric field defects and disc pallor. It is characterized by localized thickening of the arachnoid matter surrounding optic chiasma and the intracranial part of the optic nerve. There is significant inflammation, oedema, vasculitis, infarction and expansion of tuberculomas following the release of mycobacterial proteins that often lead to severe vision loss [16]. Younger age group, female gender and patients with raised CSF protein (1 g/L) are the most susceptible [33]. Interestingly, this phenomenon is rare in HIV-infected individuals. It is often difficult to differentiate the paradoxical response from disease relapse where neuroimaging might be helpful.
Cortical blindness
Cortical blindness may be seen in patients with basilar artery involvement, enlarging hydrocephalus and infarcts or tuberculomas involving the post-geniculate part of a visual pathway like optic radiation and visual cortex. The diagnosis of cortical blindness is difficult clinically as the patient has minimal signs in the presence of severe vision loss. The disc appears healthy, and pupillary reactions are normal. Homonymous hemianopia in perimetry points to posterior visual pathway involvement. But often there may be severe bilateral vision loss that precludes visual field assessment. Neuroimaging is mandatory to confirm the diagnosis in such cases.
Toxic optic neuropathy
Toxic optic neuropathy is another concerning cause of vision loss associated with the use of anti-tubercular drugs. Ethambutol, isoniazid, linezolid, streptomycin and fluoroquinolones are the main culprits. Renal insufficiency, smoking and old age are some of the risk factors that predispose to toxicity and irreversible vision loss.
Ethambutol-induced toxic optic neuropathy is dose- and duration-dependent [34] with the incidence ranging from 1 to 6% that usually presents after 4–12 months of therapy. Ethambutol [dextro-2,2′-(ethylene-diiminol-di-1-butanol dihydrochloride] is a racemic mixture of the Dextro, Meso and Levo enantiomers. The anti-tubercular activity is known to vary considerably depending on the form, the dextro being the most active and the levo the least. The dextro form also has the least ocular toxicity compared to the racemate form [35,36,37]. Walsh et al. has described two forms of ethambutol-induced toxic optic neuropathy. The central or axial form that characteristically affects the papillomacular bundle and therefore is associated with reduced visual acuity, central scotomas and red-green colour defects. The peripheral or periaxial toxicity affects the periaxial fibres leading to peripheral field defects with little or no decrease in visual acuity and colour perception [36]. The ethambutol toxicity is either related to the chelating property of the drug that adversely affects the mitochondrial function and causes neurotoxicity, or caused by the demyelination of the visual pathway. However, in the early stages, the most affected cells are the amacrine and bipolar cells in the inner plexiform layer. This leads to the weakening of the inhibitive interactions between opponent colour mechanisms [38]. The condition often presents as bilateral painless, progressive, symmetrical vision loss with variable optic nerve head pallor and central or centrocaecal scotomas. Dyschromatopsia is one of the earliest signs of toxic optic neuropathy. Blue colour vision defects can be detected in early toxicity where patients still maintain good foveolar fixation. In the advanced stage when the patient fixes eccentrically, red-green colour defects also set in [39]. Central scotomas are the common visual field defect, but bitemporal defects and peripheral field constriction have also been reported [40]. Sometimes in the absence of apparent features of toxic optic neuropathy, subclinical damage can still occur as evidenced by increased visually evoked response (VER) latency and reduced retinal nerve fibre layer (RNFL) and ganglionic cell-inner plexiform layer (GCIPL) thickness [41, 42]. In fact, the macular fibres are most sensitive to the toxic insult as reflected by the significant loss of temporal RNFL [43, 44].
Isoniazid toxicity may be associated with bilateral optic disc swelling [45] or atrophy [46] and bitemporal hemianopia. Streptomycin is known to cause pseudotumor cerebri and optic neuritis. Linezolid is mostly used in drug-resistant cases of TB. Its toxicity is associated with prolonged use (>28 days) [47] that leads to the disruption of the mitochondrial oxidative phosphorylation and protein synthesis [48]. Patients can present with disc pallor or oedema. Vision usually improves in most cases of toxic optic neuropathy after stopping the concerned drug, although irreversible vision loss has also been reported.
The differentiation among various causes of vision loss in TB becomes imperative as the management will widely vary. This often becomes challenging for the ophthalmologist or a neuro-ophthalmologist. Based on the clinical hints like time of vision loss (before or after the ATT), nature of vision loss (acute, subacute, chronic), laterality (unilateral or bilateral), pupillary involvement, disc features and type of visual field defects one can localize the probable cause and site of involvement. This can further be confirmed on neuroimaging. Therefore, meticulous clinical work-up and adjunctive neuroimaging will help you arrive at a diagnosis in most cases. Unfortunately, despite best efforts, permanent visual impairment and blindness are known to occur in TB [49].
Cranial nerve palsy
Other neuro-ophthalmologic complications that can occur in TB are cranial nerve palsies, pupillary anomalies and accommodation abnormalities. Cranial neuropathies (other than optic neuropathy) have been reported in 20–52% cases of TBM in several series. [7, 50,51,52] Abducent nerve is most frequently affected followed by the oculomotor nerve [44]. Bilateral ptosis has been reported as a manifestation in midbrain tuberculoma [53]. Trochlear and trigeminal nerve involvement are less common. Facial nerve palsy or rarely hemifacial spasms may be seen in a pontine lesion [44]. Multiple cranial nerve palsies may be seen with cavernous sinus involvement. Cranial nerves can be affected anywhere in their intracranial course, but they are most susceptible in the subarachnoid space at the base of the brain. The damage is by the mechanical compression by thick exudates/tuberculomas, by direct infectious infiltration or vasculitis of the supplying blood vessel. Abducent nerve palsy can also be caused by raised intracranial pressure. Inter-nuclear ophthalmoplegia and hemifacial spasms may sometimes be seen in a patient with rapidly progressive meningitis. Other rare manifestations like conjugate deviation of eye and vestibular nystagmus may be seen in severe disease associated with the involvement of mesencephalon or vestibulocerebellar pathways (in brain stem and cerebellum), respectively. There can be pupillary and accommodation disturbances. Afferent and efferent pupillary defects can be seen depending on the site of the lesion. Damage to the afferent visual pathway will exhibit RAPD and efferent pathway like mesencephalon, oculomotor nerve or ciliary ganglion involvement will cause anisocoria. Horner syndrome may be seen if the oculosympathetic pathway is disturbed [44].
Other features
Gaze palsies and pursuit and saccade anomalies may be associated with the involvement of the respective centres in the brain. Anton syndrome (visual anosognosia, i.e. denial of loss of vision) and Balint syndrome (difficulty in visual and spatial coordination) have been rarely described with occipital lobe involvement in CNS-TB [49]. Palinopsia, the persistent occurrence of the visual image even after the removal of stimulus and visual hallucinations have been reported with occipital lobe tuberculomas [54].
Rarely Gradenigo syndrome has been described in association with tuberculous involvement of petrous bone, characterized by periorbital unilateral pain (trigeminal nerve involvement), diplopia (sixth nerve palsy) and lower motor neuron type of facial palsy [55]. Another rare manifestation described in association with pulmonary TB is neuro-myelitis optica (NMO). The diagnosis of pulmonary TB precedes the onset of NMO. This is most likely caused by immune-mediated inflammatory demyelination of the optic nerves and spinal cord triggered by Mycobacterium tuberculosis [56]. The diagnosis of NMO is confirmed by detecting anti-aquaporin-4 antibodies in the serum. These patients may also have repeated vomiting due to area postrema involvement, altered sleep–wake cycle and other symptoms related to diencephalon involvement [57].
Management
The management of TB and its neuro-ophthalmic manifestations require multispecialty care with integrated efforts of infectious disease expert, neurologist, pulmonologist and neuro-ophthalmologist. The approach to such cases will entail the conduction of the necessary tests for confirmation of diagnosis followed by prompt treatment to minimize the visual and systemic morbidity.
Diagnosis
Laboratory investigations
The laboratory investigations include routine haematological tests like complete blood count, erythrocytes sedimentation rate, blood sugar, liver and renal function tests. All patients should be tested for HIV using an enzyme-linked immunosorbent assay considering the rising number of HIV co-infection in TB. Mantoux test may provide evidence for previous tuberculous infection, although the test lacks sensitivity and specificity especially in a vaccinated population (BCG vaccine). Chest X-ray may reveal any active or healed lung infection. Sputum examination may be done for acid-fast bacilli (AFB) in symptomatic cases. Due to heterogeneity in presentation, there are no established diagnostic criteria for tuberculous optic neuropathy and ocular TB. However, one may follow the simple guidelines given by Davis et al. that include consistent clinical signs, positive Mantoux reaction, positive IFN-γ release assay, tuberculous lesion on chest imaging, identification of AFB by microscopy or culture of extraocular tissue samples and positive response to ATT [21].
CSF analysis
CSF analysis helps in establishing the diagnosis in patients with evidence of CNS-TB. Lumbar puncture is usually performed by the treating neurologist. CSF manometry at the time of lumbar puncture evaluates the CSF opening pressure. CSF features like pleocytosis (more than 20 cells, more than 60% lymphocytes), moderately elevated proteins (more than 100 mg/dl) and low sugar (<60% of corresponding blood sugar) usually go in favour of tuberculous infection; malignancy should be ruled out by microscopic examination. Rarely, a brief neutrophilic predominance in CSF may suggest an early stage of infection or paradoxical response to ATT [58]. Definitive diagnosis is based on the isolation of tubercle bacilli in CSF smears using Ziehl–Neelsen staining (bent or curved rods that stain bright red) or CSF culture on Lowenstein Jensen media. Cultures also allow for drug sensitivity testing. But the bacterial growth is extremely slow on culture and may be evident after 3–8 weeks. The bacterial yield is known to be low in CSF samples that can be improved by centrifugation [9] and by using ventricular and cisternal CSF samples [51]. Liquid culture media like Mycobacterium growth indicator tube (Bactec) offers improved sensitivity over a solid culture that can indicate the growth by colour change within few days, although documenting a negative report sometimes requires ~21–42 days [59].
Brain biopsy
Biopsy remains the gold standard for diagnosing intracranial tuberculomas or brain abscess. Stereotactic brain biopsy is preferred over craniotomy due to reduced surgical risk and fewer complications. However, the role of biopsy should be evaluated on a case-to-case basis and considered only when deemed necessary. It should preferably be avoided in immunocompromised states like HIV or malignancy [60].
Nucleic acid amplification test (NAAT)
Currently WHO as well as INDEX TB (guidelines for extrapulmonary TB in India) [61] guidelines focus mainly on establishing definite microbiological diagnosis by isolating the tubercular bacilli and to ensure maximum yield in isolating the bacilli. NAAT has also become a part of these guidelines. NAAT is useful for the rapid detection of TB and drug resistance. Xpert MTB/RIF, a cartridge-based real-time polymerase chain reaction (PCR) test with a rapid turn-around time (around 2 h) is one of the most commonly used NAAT with a sensitivity of around 70–80% in CNS-TB [62]. It can also detect rifampicin resistance. It has been endorsed by WHO especially in the detection of multidrug-resistant (MDR) and HIV-infected TB [62]. This can be used as an adjunctive test in the diagnosis of TBM but a negative test does not rule out its diagnosis [61]. Line probe assay is another rapid PCR-based technique that can detect drug sensitivity to rifampicin and isoniazid. But the diagnostic accuracy of this test is not well-established in CNS-TB.
Interferon-γ and adenosine deaminase assays
Interferon-γ assays (ELISPOT, Quantiferon Gold) measure the person’s immune reactivity to mycobacteria based on the identification of interferon-γ released by the host T cells in response to infection. They are fast (<24 h) and do not show false positive response due to previous BCG vaccination. But it cannot distinguish between latent and active TB. Chemical assays like adenosine deaminase (ADA 2 isoform) is based on the increased production of this enzyme due to lymphocytic proliferation. Raised ADA levels were found to be a predictor of poor neurological outcomes in paediatric TBM [63]. However, there are no established cutoffs for the test (range of 5–15 IU/l). These assays are not routinely used for the diagnosis of TBM.
Neuroimaging
Neuroimaging has immensely contributed to the diagnostic ability in CNS-TB. Gadolinium-enhanced magnetic resonance imaging of the brain, orbits and occasionally spine is the modality of choice to evaluate the location and extent of the lesion. Key imaging features suggestive of TBM are leptomeningeal enhancement, basal exudates, tuberculomas, communicating or non-communicating hydrocephalous, and cerebral infarcts (Fig. 1A–D).
A Basal exudates: axial post gadolinium T1-weighted image showing smooth thin enhancement of the prepontine cistern (arrow). The enhancement is also seen along the cerebellar sulci (dashed arrow); B basal exudates: sagittal post gadolinium T1-weighted image showing smooth enhancement of the basal cisterns (arrows). Enhancement is also seen along the cerebellar sulci (dashed arrow); C exuberant basal exudates: axial post gadolinium T1-weighted image showing nodular enhancement of the perimesencephalic cisterns (arrows) and mass effect on the optic chiasma (dashed arrow); D basal exudates with tuberculomas: axial post gadolinium T1-weighted image showing smooth thin enhancement of the perimesencephalic cisterns (arrow) and multiple ring-enhancing granulomas (dashed arrow).
Tuberculomas can develop anywhere in the brain. However, it is typically infratentorial in patients aged ≤20 years. No such predilection is noted in older patients where it can be found in both infra as well as supratentorial locations [60]. Intracranial tuberculomas have variable radiological features. Bernaerts et al. have vividly described the radiological features of intracranial tuberculomas in three different stages of development: non-caseating, caseating with a solid centre and caseating with a liquid centre [64]. On MRI, non-caseating tuberculomas are hypointense relative to brain tissue in T1-weighted images and hyperintense in T2-weighted images and show homogenous enhancement with contrast. They are frequently surrounded by a halo of contiguous vasogenic white matter oedema in the early stage of the lesion (Fig. 2A–D).
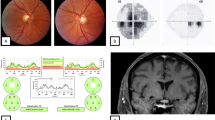
A Suprachiasmatic tuberculomas: Axial post gadolinium T1-weighted image showing disc-like enhancing tuberculomas in the suprachiasmatic location (arrows) and causing mass effect on the optic chiasma (dashed arrow); B suprachiasmatic tuberculomas mimicking a mass: coronal post gadolinium T1-weighted image showing coalescent enhancing tuberculomas in the suprachiasmatic location (arrow) mimicking a mass causing compression of the underlying optic chiasma (dashed arrow). C Fundus picture of the right eye of the same patient showing choroidal tubercle in the supero-temporal macula (arrow). D The posterior segment OCT of the same eye showing a choroidal bump with a contact sign (arrow).
In solid caseating lesions, on T1-weighted images, the lesion is relatively hypointense or isointense while T2-weighted images are isointense to hypointense. The rim of a caseating granuloma is strikingly hypointense on T2 and enhances on T1 gadolinium-enhanced MRI (Fig. 3A–B). Caseating granuloma with central liquefaction will show a hypointense core on T1 with intense rim enhancement with gadolinium. On T2 it is hyperintense and at this stage, tuberculous lesions may be indistinguishable from a pyogenic abscess on MR imaging [64].
A Axial FLAIR image showing a tuberculoma in the midbrain with characteristic central hypointense signal (arrow) and extensive surrounding oedema involving the periaqueductal grey matter and expected location of the left oculomotor and Edinger–Westphal nucleus; B sagittal T2-weighted image showing a lesion with characteristic central T2 hypointensity (dashed arrow) with minimal surrounding oedema in the lateral occipital cortex. C Optochiasmatic arachnoiditis: axial post gadolinium T1-weighted image showing smooth thin enhancement along the lining of optic chiasma and bilateral optic nerves (arrow). The enhancement is also seen along with the basal cisterns (dashed arrow). D Typical findings of TB: coronal post gadolinium T1-weighted image showing smooth thin enhancement of the basal cisterns (arrow) with hydrocephalus (asterisk) and gyriform enhancement along the right temporal lobe (dashed arrow, secondary to a subacute infarct).
MRI is useful for the early detection of optochiasmatic arachnoiditis [64, 65] that is characterized by perichiasmal enhancement, hypertrophy of the chiasma and the cisternal segments of optic nerves (Fig. 3C). Confluent enhancing lesions in the suprasellar cistern in the chiasmatic region have also been described [66]. In immunosuppressed patients (e.g. HIV co-infection), there is minimal or no meningeal enhancement due to impaired immune response and absent basal exudates [64].
Infarcts in CNS-TB are typically periventricular due to the involvement of lenticulostriate and thalamo-perforating arteries (Fig. 3D).
MRI can also aid in differentiation between optic neuritis and perineuritis. The latter typically has a ‘tram-track’ appearance on axial views due to enhancement around the optic nerve and a ‘doughnut’ appearance on coronal views with a streaky enhancement of orbital fat. Occasionally, the substance of the optic nerve can show enhancement in optic perineuritis due to inflammation of the intraneural pial septa and the nerve sheath [25].
While MRI provides the anatomical picture, MR spectroscopy is a technique that provides information about the metabolic profile of the tissue. Single-voxel methods like Point RESolved Spectroscopy usually provide diagnostic-quality data in a very short time [67]. MR spectroscopy has been found useful in recognizing tuberculomas by assessing the lipid lactate peak (Fig. 4) [68]. A singlet peak at ≈3.8 ppm found in the majority of tuberculomas and absent in most malignant tumours helps in differentiating the two entities. Also, a higher Cho/Cr (choline/creatine) and mI/Cr (myo-inositol/ creatine) ratios favour malignant lesions over tuberculomas [69].
Contrast-enhanced computed tomography (CECT) could be an alternative in case of non-availability of MRI. This is especially useful in detecting bone infections or meningeal calcifications in resolving cases. The presence of a triad of basal enhancement, hydrocephalus and infarcts on CT have 100% diagnostic specificity for TBM in children [70]. Early tuberculomas are hypodense initially on CT. But as the capsule develops around them, they become isodense with ring enhancement on contrast. After calcification, lesions become hyperdense and show little enhancement. They are round or oval and usually solitary but multiple lesions may be seen in different stages of progression. The non-caseating granuloma is slightly hypodense or isodense and enhances homogeneously with contrast. In solid caseating lesions, the central portion enhances heterogeneously while the capsule presents a ring-enhancing pattern, which tends to be unbroken and of uniform thickness. Caseating granuloma with central liquefaction can be seen as a hypodense core surrounded by a dense ring of enhancement on CECT [64].
A tuberculous lesion on CT needs to be differentiated from other granulomatous lesions like sarcoidosis, neoplasms and parasitic infestations like cysticercosis or toxoplasmosis [15]. MRI is superior to CT scan in picking up small lesions and delineating lesions especially in the lower brain stem or posterior fossa [15].
Ophthalmic work-up
In the presence of neuro-ophthalmic features, a thorough ophthalmic work-up becomes mandatory. In case of visual function defects or field loss, distance and near best-corrected visual acuity, colour vision, contrast sensitivity and visual fields should be evaluated. In toxic optic neuropathy, a 100-hue test is more sensitive for colour assessment as it can detect early subtle changes and blue-yellow defects that may be missed on Ishihara test type. Static (Humphrey) or kinetic (Goldmann) perimetry should be done for assessment of the type of visual field defect. In patients with diplopia, paralytic work-up including diplopia charting and Hess charting should be done at baseline and follow-up. Peripapillary RNFL on optical coherence tomography (OCT) at presentation and on follow-up helps in evaluating the disease course in disc oedema and atrophy. OCT can quantify RNFL [71] and retinal ganglion cell [72] loss in early ethambutol toxicity before the fundus changes become apparent. Menon et al. [43] showed that assessment of P100 wave latency of pattern-VER and RNFL thickness on OCT can help detect subclinical ethambutol toxicity and therefore these tests should be conducted especially in patients who are prescribed >15–20 mg/kg/day of ethambutol for >2 months. VEP also gives an idea about the intactness of the visual pathway and visual cortex, in cases presenting with severe vision loss or cortical blindness. Recently, optic nerve sheath diameter monitoring using ultrasound has been described as a non-invasive method of detecting raised intracranial pressure in cases of CNS-TB and the results correlate positively with invasive intracranial pressure catheter monitoring [73].
Treatment
The treatment is primarily aimed at eliminating the infection by the use of anti-tubercular drugs and managing the associated features like raised intracranial pressure and inflammation. Multidrug therapy of bactericidal drugs isoniazid, rifampicin, pyrazinamide and ethambutol administered for 2 months (intensive phase) followed by isoniazid and rifampicin for another 7–10 months (continuation phase) is recommended for the treatment of TBM in immunocompetent patients [74]. Similar regimen is used in paediatric cases with TBM [75]. The patient must be regularly evaluated for side effects like hepatotoxicity and toxic optic neuropathy during the course of ATT. In CNS-TB with visual impairment often ethambutol is replaced with ofloxacin, levofloxacin or streptomycin as a first-line drug to prevent further visual deterioration. Isoniazid and pyrazinamide, administered orally in a dosage of 10 and 35–45 mg/kg, respectively, are bactericidal and can well penetrate both inflamed and uninflamed meninges thereby achieving CSF concentration above the minimum inhibitory concentration (MIC) for Mycobacterium tuberculosis. The CSF concentration of oral rifampicin (10 mg/kg), intramuscular streptomycin (20–40 mg/kg) and oral ethambutol are equal to or slightly above the MIC of Mycobacterium tuberculosis and they do not penetrate uninflamed meninges. Oral ethionamide (15–20 mg/kg) penetrates both healthy and inflamed meninges and has a CSF concentration slightly above the MIC for Mycobacterium tuberculosis. While low-dose ethambutol (15 mg/kg) and ethionamide are bacteriostatic, rifampicin, streptomycin and high dose ethambutol (25 mg/kg) are bactericidal. The optimum dose and duration of chemotherapy are not defined. Therefore, treatment should be modified based on the change in CSF fluid parameters (like cell count, glucose, protein, etc.) as noted on repeated lumbar punctures.
Intermittent ATT regimen should be avoided especially in HIV co-infected patients due to the higher risk of relapse and emergence of drug resistance. Anti-retroviral therapy (ART) that is ideally started within 2 weeks of initiation of ATT if CD4+ count is <50 cell/µL and by 8–12 weeks if CD4 count is ≥50 µ/L in pulmonary TB with HIV co-infection, should be avoided in the first 8 weeks of ATT in TBM. The clinicians must keep in mind that concurrent administration of ART and ATT can cause paradoxical worsening of clinical manifestations of TB. This phenomenon results from the reconstitution of immune responsiveness after the initiation of ART known as immune reconstitution inflammatory syndrome [74]. Also, administration of rifampicin decreases the concentration of ART, which may require dose adjustment when given together.
The US Centres for Disease Control and American Thoracic Society recommends [74] adjunctive use of low-dose systemic corticosteroids in CNS-TB. Its anti-inflammatory effect reduces cerebral oedema, vasculitis and the deleterious effects of immune response in CNS and ocular tissue and therefore lowers mortality [76]. Oral prednisolone is started in a dose of 1 mg/kg/day and gradually tapered off over 4–8 weeks. Oral dexamethasone can be used as an alternative. However, steroids must always be used in conjunction with ATT and never alone as it may flare-up a latent systemic infection. Significant reduction in mortality and the incidence of permanent neurological sequelae have been observed with steroids. However, stopping or tapering the steroids prematurely may lead to paradoxical enlargement or appearance of tuberculomas in some patients usually in the initial 3 months of discontinuation of the drug. One possible mechanism is that the restoration of the blood–brain barrier by ATT decreases the penetration of anti-tubercular drugs into the brain. However, this theory does not hold true for cases treated with isoniazid and pyrazinamide as these drugs can cross the non-inflamed meninges. A more accepted mechanism is the enhanced delayed-type hypersensitivity that leads to the activation and accumulation of lymphocytes and macrophages at the site of bacillary deposition or toxin production when the bacilli die. If this activation occurs at the site of microscopic foci then tuberculoma appears and if it occurs at the site of macroscopic tuberculoma then there is enlargement [15]. Therefore timing, dose and duration of steroid therapy are very crucial. Tuberculous optochiasmatic arachnoiditis and tuberculomas including the paradoxical reaction are generally managed medically using a similar regimen of ATT in conjunction with steroids. In case of a life-threatening visual complication associated with optochiasmatic arachnoiditis, pulse therapy with intravenous methylprednisolone should be considered for 3–5 days followed by oral steroids.
Immuno-modulating drugs like thalidomide are used as a back-up option in cases of TBM and optochiasmatic arachnoiditis not responding to the above therapy. Thalidomide exhibits anti-inflammatory properties and acts by inhibiting TNF-alpha secretion by monocytes and macrophages [77,78,79]. Remarkable visual recovery has been demonstrated in patients with optochiasmatic arachnoiditis following thalidomide therapy [37]. But its use is limited by the risk of associated severe birth defects. Infliximab has shown encouraging result in corticosteroid-resistant paradoxical optochiasmatic arachnoiditis [80].
As toxic optic neuropathy is largely preventable, it is crucial to monitor closely the status of the optic nerve using tests like colour vision, contrast sensitivity, VER, OCT and visual fields in patients on long-term ATT. Most cases respond to stopping the culprit drug. For ethambutol-induced toxic optic neuropathy, there is no other specific treatment and visual recovery may be noted over weeks to months after discontinuation of the drug. However, the vision may still deteriorate or fail to recover when the damage is severe enough. To prevent isoniazid induced optic neuropathy, pyridoxine 25–100 mg/day may be added [71]. It may stabilize or even reverse the toxicity although the improvement may be due to the stoppage of the drug itself. Therefore, the first step that should be taken promptly at the slightest suspicion of ATT-related toxic optic neuropathy is to stop the offending drug as any delay can lead to irreversible vision loss. As both ethambutol and isoniazid are used concurrently in the treatment of TB, if the stoppage of one drug does not lead to improvement in the patient’s visual acuity then the other drug should also be stopped. Nevertheless, stopping the drug should always be done in consultation with an infectious disease expert [71, 81].
TBM with hydrocephalus is mostly managed medically with the combination of ATT, steroids and dehydrating agents like acetazolamide, furosemide and mannitol [82]. Surgical intervention is considered when medical therapy fails. But before planning surgery, one must reconsider the diagnosis and rule out other differentials in case of non-responsiveness to ATT. Ventriculoperitoneal shunt surgery is considered in cases with progressively deteriorating vision or sensorium as seen in advanced stages of TBM with hydrocephalus. The shunt relieves the CSF pressure by draining the CSF. We must keep in mind that post-operatively patient can develop vision loss due to shunt failure, shunt malposition, associated haemorrhage or shunt infection. Shunt malfunction causes raised intracranial pressure, papilledema and consequent secondary optic atrophy. The risk of shunt obstruction and failure is higher in TBM patients due to higher protein and cellular content in the CSF. Hyperfunctioning shunt with low CSF pressure can also result in rapid vision loss by causing optic chiasmal prolapse in a partially empty sella [29]. This complication is rare with programmable shunts with adjustable external valve that regulates the CSF flow. Shunt surgery carries a poor prognosis in HIV-infected cases and therefore should be avoided in them [83]. Endoscopic third ventriculostomy (ETV) can be considered as an alternate procedure that avoids insertion of shunt and its related complications. However, it must be avoided in acute cases where the subarachnoid space is obliterated with exudates and the floor of the third ventricle is coated with tubercles and granulation tissue, thus increasing the risk of bleed [84]. CSF leak and intraoperative bleed are some of the complications reported with ETV. Neurosurgical decompression of optic chiasma by excision of the optochiasmatic tuberculoma has been described in certain cases where corticosteroids have failed to improve vision [85, 86].
Outcome
The presence of neuro-ophthalmic manifestations influences the disease outcome in TB. Factors predictive of poor visual outcome are low presenting visual acuity, diplopia, hydrocephalus, colour vision defects, optic atrophy, papilledema, presence of abducent nerve palsy, evidence of optochiasmatic arachnoiditis and optochiasmatic tuberculoma on imaging, miliary TB, modified Barthel index score ≤12, RNFL defects on OCT and raised CSF protein (1 g/L) [7, 8]. Low visual acuity (<6/18) [7], complete ophthalmoplegia [6], papilledema [87], cranial nerve VI and VII palsy [8] are some of the neuro-ophthalmic features associated with higher mortality in TBM. This underscores the impact of neuro-ophthalmic features in determining the systemic outcome in these patients.
Conclusion
Neuro-ophthalmic features in TB have myriad presentations that often pose a diagnostic dilemma. They not only constitute an important manifestation of this disease (especially CNS-TB) but also are predictors of clinical outcome. With advanced diagnostic and neuroimaging techniques, they are being increasingly recognised. Knowledge and understanding of these manifestations can aid in early identification and timely management of the disease and thereby prevent sight as well as life-threatening complications.
Summary
What is known about this topic
-
Neuro-ophthamic features are a known association in tuberculosis (TB), especially CNS TB.
-
These features may be related to the visual pathway invovement, cranial nerve involvement, drug toxicity to ATT or immune mediated reaction to ATT.
What this study adds
-
This study summarizes the spectrum of neuro-ophthalmic features seen in TB, its etiopathogenenis, diagnosis and management.
References
Organization WH. WHO Global tuberculosis report 2019. World Health Organization. 2020. Available from: http://www.who.int/tb/publications/global_report/en/.
WHO. WHO | Tuberculosis and HIV. WHO. 2015. Available from: http://www.who.int/hiv/topics/tb/en/.
Rieder HL, Snider DE Jr, Cauthen GM. Extrapulmonary tuberculosis in the United States. Am Rev Respir Dis. 1990;141:347–51.
CDC. Extrapulmonary tuberculosis cases and percentages by site of disease: reporting areas. Atlanta, GA: Centers Disease Control and Prevention; 2005.
Berger JR. Tuberculous meningitis. Curr Opin Neurol 1994;7:191–200.
Amitava AK, Alam S, Hussain R. Neuro-ophthalmic features in pediatric tubercular meningoencephalitis. J Pediatr Ophthalmol Strabismus. 2001;38:229–34.
Sinha MK, Garg RK, Anuradha HK, Agarwal A, Singh MK, Verma R. et al. Vision impairment in tuberculous meningitis: predictors and prognosis. J Neurol Sci. 2010;290:27–32. https://doi.org/10.1016/j.jns.2009.12.012.
Verma R, Sarkar S, Garg RK, Malhotra HS, Sharma PK, Saxena S. Ophthalmological manifestation in patients of tuberculous meningitis. QJM. 2019;112:409–19.
Dastur HM. Diagnosis and neurosurgical treatment of tuberculous disease of the CNS. Neurosurg Rev. 1983;6:111–7.
Garg RK, Paliwal V, Malhotra HS. Tuberculous optochiasmatic arachnoiditis: a devastating form of tuberculous meningitis. Expert Rev Anti Infect Ther. 2011;9:719–29.
Paige C, Bishai WR. Penitentiary or penthouse condo: the tuberculous granuloma from the microbe’s point of view. Cell Microbiol. 2010;12:301–9. https://doi.org/10.1111/j.1462-5822.2009.01424.x. Accessed 10 May 2020.
Gray F, Duyckaerts C, de Girolami U. Escourolle and Poirier’s manual of basic neuropathology. Oxford University Press, Oxford; 2018. https://doi.org/10.1093/med/9780190675011.001.0001.
Aulakh R, Chopra S. Pediatric tubercular meningitis: a review. J Pediatr Neurosci. 2018;13:373–82. Available from: /pmc/articles/PMC6413593/?report=abstract. Accessed 22 Jul 2020.
Teoh R, Poon W, Humphries MJ, O’Mahony G. Suprasellar tuberculoma developing during treatment of tuberculous meningitis requiring urgent surgical decompression. J Neurol. 1988;235:321–2.
Afghani B, Lieberman JM. Paradoxical enlargement or development of intracranial tuberculomas during therapy: case report and review. Clin Infect Dis. 1994;19:1092–9.
Kalkan A, Serhatlioglu S, Ozden M, Denk A, Demirdag K, Yilmaz T, et al. Paradoxically developed optochiasmatic tuberculoma and tuberculous lymphadenitis: a case report with 18-month follow up by MRI. South Med J. 2006;99:388–92.
Van Crevel R, Kleinnijenhuis J, Oosting M, Joosten LAB, Netea MG. Innate immune recognition of mycobacterium tuberculosis. Clin Dev Immunol. 2011;2011:405310.
Mishra M, Rath S, Acharya B, Mohanty S, Panigrahi B. Neuro-ophthalmic profile in TBM. Ind J Tub. 1985;23:142.
Mooney AJ. Some ocular sequelae of tuberculous meningitis: a preliminary survey, 1953–4. Am J Ophthalmol. 1956;41:753–68. https://doi.org/10.1016/0002-9394(56)91768-8.
Gupta V, Gupta A, Rao NA. Intraocular Tuberculosis-An Update. Surv Ophthalmol 2007;52:561–87.
Davis EJ, Rathinam SR, Okada AA, Tow SL, Petrushkin H, Graham EM, et al. Clinical spectrum of tuberculous optic neuropathy. J Ophthalmic Inflamm Infect. 2012;2:183–9.
Narayanan S, Prakash D, Subramaniam G. Bilateral primary optic neuropathy as the presenting manifestation of tuberculosis in an immunocompetent patient. IDCases. 2019;18:e00579. https://doi.org/10.1016/j.idcr.2019.e00579.
Jacob M, Kodjikian L, Ponceau B, Grange JD. La névrite péri-optique: complication méconnue de la tuberculose? J Français d'Ophtalmologie. 2006;29:328.e1–328.e5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16557166. Accessed 11 May 2020.
Ryu WY, Kim JS. Optic perineuritis simultaneously associated with active pulmonary tuberculosis without intraocular tuberculosis. Int J Ophthalmol. 2017;10:1477–8.
Purvin V, Kawasaki A, Jacobson DM. Optic perineuritis: clinical and radiological features. Arch Ophthalmol. 2001;119:1299–306. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11545635. Accessed 11 May 2020.
Murugan S, Sundaralakshmi P. Commentary: tuberculosis in neuro ophthalmology—how different it is? Indian J Ophthalmol. 2019;67:1212–4.
Leonard JM, Des Prez RM. Tuberculous meningitis. Infect Dis Clin N Am. 1990;4:769–87.
Abbas A, Shukla R, Ahuja RC, Gupta RK, Singh KD, Saxena S. Visual impairment in HIV negative tuberculosis meningitis. J Meningitis. 2015;1:1–7.
Moghekar AR. Neuro-ophthalmic manifestations in adult hydrocephalus. Int Ophthalmol Clin. 2014;54:115–21.
Murthy JMK. Management of intracranial pressure in tuberculous meningitis. Neurocrit Care 2005;2:306–12.
Raut T, Garg RK, Jain A, Verma R, Singh MK, Malhotra HS. et al. Hydrocephalus in tuberculous meningitis: Incidence, its predictive factors and impact on the prognosis. J Infect. 2013;66:330–7. https://doi.org/10.1016/j.jinf.2012.12.009.
Hejazi N, Hassler W. Multiple intracranial tuberculomas with atypical response to tuberculostatic chemotherapy. Review of the literature and own experience. Acta Neurochir. 1997;139:194–202.
Anupriya A, Sunithi M, Maya T, Goel M, Alexander M, Aaron S, et al. Tuberculous optochiasmatic arachnoiditis. Neurol India. 2010;58:732–5.
Grzybowski A, Zülsdorff M, Wilhelm H, Tonagel F. Toxic optic neuropathies: an updated review. Acta Ophthalmol. 2015;93:402–10. Available from: https://pubmed.ncbi.nlm.nih.gov/25159832/. Accessed 21 Sep 2020.
Wilkinson RG, Shepherd RG, Thomas JP, Baughn C. Stereospecificity in a new type of synthetic antituberculous agent. J Am Chem Soc. 1961;83:2212–3.
Leibold JE. The ocular toxicity of ethambutol and its relation to dose. Ann N Y Acad Sci. 1966;135:904–9. Available from: https://pubmed.ncbi.nlm.nih.gov/5220245/. Accessed 30 Jul 2020.
Kahana LM. Toxic ocular effects of ethambutol. Can Med Assoc J. 1987;137:213–6. Available from: /pmc/articles/PMC1492367/?report=abstract. Accessed 30 Jul 2020.
Van Dijk BW, Spekreijse H. Ethambutol changes the color coding of carp retinal ganglion cells reversibly. Investig Ophthalmol Vis Sci. 1983;24:128–33.
Polak BCP, Leys M, Van Lith GHM. Blue yellow colour vision changes as early symptoms of ethambutol oculotoxicity. Ophthalmologica 1985;191:223–6. Available from: http://library1.nida.ac.th/termpaper6/sd/2554/19755.pdf.
Behbehani R. Clinical approach to optic neuropathies. Clin Ophthalmol. 2007;1:233–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19668477. Accessed 30 Apr 2020.
Mandal S, Saxena R, Dhiman R, Mohan A, Padhy SK, Phuljhele S, et al. Prospective study to evaluate incidence and indicators for early detection of ethambutol toxicity. Br J Ophthalmol. 2020:bjophthalmol-2020-316897. Available from: http://bjo.bmj.com/lookup/doi/10.1136/bjophthalmol-2020-316897. Accessed 30 Jul 2020.
Koul PA. Ocular toxicity with ethambutol therapy: timely recaution. Lung India. 2015;32:1–3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4298909/. Accessed 22 Jul 2020.
Menon V, Jain D, Saxena R, Sood R. Prospective evaluation of visual function for early detection of ethambutol toxicity. Br J Ophthalmol. 2009;93:1251–4. Available from: https://pubmed.ncbi.nlm.nih.gov/19525243/. Accessed 22 Jul 2020.
Miller NR, Walsh FB. In: Frank B, Hoyt WF, editors. Walsh and Hoyt’s clinical neuro-ophthalmology. Lippincott Williams & Wilkins, Philadelphia; 2005.
Van Stavern GP, Newman NJ. Optic neuropathies. An overview. Ophthalmol Clin North Am. 2001;14:61–71.
Kass I, Mandel W, Cohen H, Dressler SH. Isoniazid as a cause of optic neuritis and atrophy. J Am Med Assoc. 1957;164:1740–3. https://doi.org/10.1001/jama.1957.02980160012003.
De Vriese AS, Van Coster R, Smet J, Seneca S, Lovering A, Van Haute LL, et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis. 2006;42:1111–7.
Javaheri M, Khurana RN, O’Hearn TM, Lai MM, Sadun AA. Linezolid-induced optic neuropathy: a mitochondrial disorder? Br J Ophthalmol. 2007;91:111–5.
Nataprawira HM, Ruslianti V, Solek P, Hawani D, Milanti M, Anggraeni R, et al. Outcome of tuberculous meningitis in children: the first comprehensive retrospective cohort study in Indonesia. Int J Tuberc Lung Dis. 2016;20:909–14.
Thwaites GE, Hien TT. Tuberculous meningitis: many questions, too few answers. Lancet Neurol. 2005;4:160–70.
Verdon R, Chevret S, Laissy J-P, Wolff M. Tuberculous meningitis in adults: review of 48 cases. Clin Infect Dis. 1996;22:982–8.
Kent SJ, Crowe SM, Yung A, Lucas CR, Mijch AM. Tuberculous meningitis: a 30-year review. Clin Infect Dis Publ Infect Dis Soc Am. 1993;17:987–94.
Kumar S, Rajshekher G, Prabhakar S. Isolated bilateral ptosis as the presentation of midbrain tuberculoma. Neurol India. 2008;56:212–3.
Werring DJ, Marsden CD. Visual hallucinations and palinopsia due to an occipital lobe tuberculoma. J Neurol Neurosurg Psychiatry. 1999;66:684.
Motamed M, Kalan A. Gradenigo’s syndrome. Postgrad Med J. 2000;76:559–60. Available from: www.postgradmedj.com. Accessed 22 Jul 2020.
Zatjirua V, Butler J, Carr J, Henning F. Neuromyelitis optica and pulmonary tuberculosis: a case-control study. Int J Tuberc Lung Dis. 2011;15:1675–9.
Chitnis T, Ness J, Krupp L, Waubant E, Hunt T, Olsen CS, et al. Clinical features of neuromyelitis optica in children: US Network of Pediatric MS Centers report. Neurology. 2016;86:245–52. Available from: /pmc/articles/PMC4733158/?report=abstract. Accessed 22 Jul 2020.
Sütlaş PN, Ünal A, Forta H, Şenol S, Kırbaş D. Tuberculous meningitis in adults: review of 61 cases. Infection. 2003;31:387–91. Available from: http://link.springer.com/10.1007/s15010-003-3179-1. Accessed 23 May 2020.
Ardito F, Posteraro B, Sanguinetti M, Zanetti S, Fadda G. Evaluation of BACTEC Mycobacteria Growth Indicator Tube (MGIT 960) automated system for drug susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol. 2001;39:4440–4. Available from: https://pubmed.ncbi.nlm.nih.gov/11724858/. Accessed 22 Jul 2020.
Nicolls DJ, King M, Holland D, Bala J, Del, Rio C. Intracranial tuberculomas developing while on therapy for pulmonary tuberculosis. Lancet Infect Dis. 2005;5:795–801.
Sharma SK, Ryan H, Khaparde S, Sachdeva KS, Singh AD, Mohan A, et al. Index-TB guidelines: guidelines on extrapulmonary tuberculosis for India. Indian J Med Res. 2017;145:448–63. Available from: /pmc/articles/PMC5663158/?report=abstract. Accessed 21 Sep 2020.
World Health Organization. Rapid implementation of the Xpert MTB/RIF diagnostic test. Technical and operational ‘How to’ practical considerations. World Health Organization. 2011. p. 1–34. Available from: http://www.who.int/about/licensing/copyright_form/en/index.html. Accessed 21 Sep 2020.
Jakka S, Veena S, Rao ARM, Eisenhut M. Cerebrospinal fluid adenosine deaminase levels and adverse neurological outcome in pediatric tuberculous meningitis. Infection 2005;33:264–6.
Bernaerts A, Vanhoenacker FM, Parizel PM, Van Goethem JWM, van Altena R, Laridon A, et al. Tuberculosis of the central nervous system: overview of neuroradiological findings. Eur Radiol. 2003;13:1876–90.
Clavier E, Thiebot J, Freger P, Mihout B, Bernet J, Benozio M. [Optochiasmatic tuberculoma and MRI. 2 cases]. J Radiol. 1988;69:333–7.
Anupriya A, Sunithi M, Maya T, Goel M, Alexander M, Aaron S, et al. Tuberculous optochiasmatic arachnoiditis. Neurol India. 2010;58:732–5.
Gupta RK, Jobanputra KJ, Yadav A. MR spectroscopy in brain infections. Neuroimaging Clin N Am. 2013;23:475–98. https://doi.org/10.1016/j.nic.2013.03.004.
Kingsley PB, Shah TC, Woldenberg R. Identification of diffuse and focal brain lesions by clinical magnetic resonance spectroscopy. NMR Biomed. 2006;19:435–62. Available from: http://doi.wiley.com/10.1002/nbm.1039. Accessed 23 May 2020.
Morales H, Alfaro D, Martinot C, Fayed N, Gaskill-Shipley M. MR spectroscopy of intracranial tuberculomas: a singlet peak at 3.8 ppm as potential marker to differentiate them from malignant tumors. Neuroradiol J. 2015;28:294–302.
Andronikou S, Smith B, Hatherhill M, Douis H, Wilmshurst J. Definitive neuroradiological diagnostic features of tuberculous meningitis in children. Pediatr Radiol. 2004;34:876–85.
Sharma P, Sharma R. Toxic optic neuropathy. Indian J Ophthalmol. 2011;59:137–41.
Gümüş A, Öner V. Follow up of retinal nerve fiber layer thickness with optic coherence tomography in patients receiving anti-tubercular treatment may reveal early optic neuropathy. Cutan Ocul Toxicol. 2015;34:212–6.
Wang L-J, Chen L-M, Chen Y, Bao L-Y, Zheng N-N, Wang Y-Z, et al. Ultrasonography assessments of optic nerve sheath diameter as a noninvasive and dynamic method of detecting changes in intracranial pressure. JAMA Ophthalmol. 2018;136:250–6.
Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A. et al. Executive summary: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63:853–67. https://doi.org/10.1093/cid/ciw566.
American Academy of Pediatrics. Red book: 2015 report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015.
Thwaites GE, Bang ND, Dung NH, Quy HT, Oanh DTT, Thoa NTC, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741–51. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa040573. Accessed 23 May 2020.
Nicolls DJ, King M, Holland D, Bala J, Del Rio C. Intracranial tuberculomas developing while on therapy for pulmonary tuberculosis. Lancet Infect Dis. 2005;5:795–801.
Roberts MTM, Mendelson M, Meyer P, Carmichael A, Lever AML. The use of thalidomide in the treatment of intracranial tuberculomas in adults: two case reports. J Infect. 2003;47:251–5.
Schoeman JF, Andronikou S, Stefan DC, Freeman N, Van Toorn R. Tuberculous meningitis-related optic neuritis: recovery of vision with thalidomide in 4 consecutive cases. J Child Neurol. 2010;25:822–8.
Blackmore TK, Manning L, Taylor WJ, Wallis RS. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis. 2008;47:e83–5.
Saxena R, Singh D. Tuberculous optic neuropathy. In: Kumar A, Chawla R, Sharma N, editors. Ocular tuberculosis. Essentials in Ophthalmology. Springer, Cham; 2017. p. 95–9. https://doi.org/10.1007/978-3-319-57520-9_10.
Rizvi I, Garg RK, Malhotra HS, Kumar N, Sharma E, Srivastava C. et al. Ventriculoperitoneal shunt surgery for tuberculous meningitis: a systematic review. J Neurol Sci. 2017;375:255–63. Available from: https://pubmed.ncbi.nlm.nih.gov/28320142/. Accessed 8 Feb 2021.
Nadvi SS, Nathoo N, Annamalai K, van Dellen JR, Bhigjee AI. Role of cerebrospinal fluid shunting for human immunodeficiency virus-positive patients with tuberculous meningitis and hydrocephalus. Neurosurgery. 2000;47:644–50–51. Available from: https://pubmed.ncbi.nlm.nih.gov/10981752/. Accessed 8 Feb 2021.
Rajshekhar V. Management of hydrocephalus in patients with tuberculous meningitis. Neurol India. 2009;57:368. Available from: http://www.neurologyindia.com/text.asp?2009/57/4/368/55572. Accessed 8 Feb 2021.
Marcus AO, Demakas JJ, Ross HA, Duick DS, Crowell RM. Optochiasmatic arachnoiditis with treatment by surgical lysis of adhesions, corticosteroids, and cyclophosphamide: report of a case. Neurosurgery 1986;19:101–3.
Navarro IM, Peralta VH, Leon JA, Varela EA, Cabrera JM. Tuberculous optochiasmatic arachnoiditis. Neurosurgery 1981;9:654–60.
Mahadevan B, Mahadevan S, Serane VT. Prognostic factors in childhood tuberculous meningitis. J Trop Pediatr. 2002;48:362–5. https://doi.org/10.1093/tropej/48.6.362.
Acknowledgements
We would like to acknowledge Dr. Saurabh Verma for helping us with the images.
Author information
Authors and Affiliations
Contributions
The authors declare that the manuscript has been read and approved by all the authors. RD and SL were responsible for designing the review protocol, writing the protocol and report, conducting the search, screening potentially eligible studies, extracting and analysing data, interpreting results, updating reference lists, creating ‘Summary of findings’ table, critical revision of the article and the final approval of the version. PKP and NH were responsible for writing the protocol and report, screening potentially eligible studies, extracting and analysing data, interpreting results and updating reference lists. SS and RS were responsible for the critical revision of the article and the final approval of the version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhiman, R., Lakra, S., Panda, P.K. et al. Neuro-ophthalmic manifestations of tuberculosis. Eye 36, 15–28 (2022). https://doi.org/10.1038/s41433-021-01619-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01619-6