Abstract
Objectives
Neuroimaging studies in patients with bipolar disorder have suggested that a neuropathological process may be effective in this disease. Neurodegenerative changes in the retina can be followed by optical coherence tomography, a non-invasive imaging method that allows in vivo visualization of the retinal layers. The aim of this study was to investigate the possible differences in optical coherence tomography parameters during euthymic, manic, and depressive episodes in patients diagnosed with bipolar disorder.
Methods
A total of 150 patients with bipolar disorder were included in the study, divided into three groups (50 patients in a euthymic state, 50 patients in a manic state, and 50 patients in a depressive state) and compared with 50 healthy controls. Ganglion cell complex thickness was measured with automated macular segmentation software of spectral-domain optical coherence tomography.
Results
Ganglion cell complex thicknesses were thicker in all quadrants in patient groups than the control group but the differences were significant in perifoveal superior and perifoveal inferior quadrants (p < 0.001, p < 0.001). There were no differences in ganglion cell complex thickness among the patient groups (p > 0.05).
Conclusion
The evaluation of ganglion cell complex thickness by spectral-domain optical coherence tomography may give a clue for monitoring neurodegenerative changes in patients with bipolar disorder.
Similar content being viewed by others
Introduction
Bipolar disorder (BD) is a mood disorder that is characterized by manic, depressive and euthymic episodes and is estimated to affect up to 1% of the global population [1, 2]. In brief, manic episode includes three or more of these symptoms with grandiosity, racing thoughts, decreased need for sleep, more talkative than usual, distractibility, increase in goal-directed activity or psychomotor agitation. Depressive episode includes five or more of these symptoms with depressed mood, marked loss of interest or feeling no pleasure in all -or almost all- activities, significant weight loss when not dieting, weight gain, or decrease or increase in appetite, insomnia or hypersomnia, psychomotor agitation or retardation, fatigue or loss of energy, feelings of worthlessness or excessive or inappropriate guilt, diminished ability to think or concentrate, or indecisiveness and thinking about, planning or attempting suicide. The euthymic period is defined as a normal mood in which the person is not depressed or expansive, between these periods [3].
Whether BD is a neurodegenerative disease remains an open question, but neuroimaging studies are now adding evidence that supports this possibility [4]. The demonstration of structural brain abnormalities in both acute and remission phases in patients diagnosed with BD further suggests that this disease may be a neuropathological process and recent neuroimaging studies have also provided further support for neurodegeneration in BD [5]. Also, it has been claimed that inflammatory and immunologic responses may have a critical role in the pathophysiology of BD [6,7,8].
Optical coherence tomography (OCT), a non-invasive imaging method that allows in vivo visualization of the retinal layers [9]. The retina and its layers are considered to be part of the central nervous system and have become an important anatomical parameter for monitoring neurodegeneration [10]. Especially, ganglion cell complex (GCC) which is the combination of the three inner retinal layers (retinal nerve fibre layer [RNFL] containing the ganglion cell axons, ganglion cell layer [GCL] containing the ganglion cell bodies, and inner plexiform layer [IPL] containing the ganglion cell dendrites) reflects the degenerative changes of the retinal ganglion cells [11]. Few studies in the literature have examined the retina in patients diagnosed with BD [12,13,14,15,16,17]. In addition, no study has yet examined the retina during manic and depressive episodes in these patients. Therefore, the aim of the present study was to investigate the possible differences in GCC thickness during euthymic, manic, and depressive episodes in patients diagnosed with BD and to compare them with GCC thickness of the healthy controls. A further goal was to determine a possible relationship between these parameters and BD severity and clinical features.
Methods
Patient and control selection
The participants were 150 patients with BD (50 with manic disease, 50 with depressive disease, and 50 with euthymic disease [in remission for at least 6 months]) who were followed up in the Psychiatry Clinic of University of Health Sciences, Konya Education and Research Hospital between May 2018 and October 2018 and who met the inclusion and exclusion criteria. A sociodemographic data form developed by the researcher was administered to each patient and to a healthy control group of 50 age- and gender-matched healthy individuals who had no psychiatric disorder according to the Structured Clinical Interview I (SCID-I). All patients were interviewed using the SCID-I to confirm the diagnosis of BD-I. The Hamilton Depression Rating Scale (HDRS) and Young Mania Rating Scale (YMRS) were then administered to the patient groups [18, 19]. Patients were considered euthymic if they scored ≤7 on both the HDRS and the YMRS, depressive if they scored >7 on the HDRS and <7 on the YMRS, and manic if they scored <7 on the HDRS and >7 on the YMRS. The Clinical Global Impression Scale (CGI) was also administered to the patients during follow-ups. The study was approved by the clinical research ethics committee of Selcuk University Faculty of Medicine (Approval Number 2018/144, Date: April 18, 2018) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Inclusion/exclusion criteria
Patients between 18 and 65 years of age and diagnosed with BD-I according to the DSM-IV-TR diagnostic criteria were included in the study. Patients diagnosed with organic bipolar disorder or any psychiatric disorders other than BD-I according to the DSM-IV-TR diagnostic criteria were excluded from the study. Patients were also excluded if they had a history of any of the following medical conditions: eye diseases (glaucoma, cataract, macular diseases, high myopia and hypermetropia, or uveitis) affecting OCT measurements; neurological, autoimmune, or systemic diseases (epilepsy, Parkinson’s Disease, Alzheimer’s disease, multiple sclerosis, diabetes mellitus, hypertension, hyperlipidemia or rheumatologic diseases) affecting the visual pathways; brain injury; a diagnosis of mental retardation; or alcohol or substance abuse.
Eye examination and OCT measurements
All participants underwent a detailed ophthalmological examination at the Ophthalmology Outpatient Clinic of University of Health Sciences Konya Training and Research Hospital to exclude eye diseases that could affect the retina. The evaluation included visual acuity testing, intraocular pressure measurement, and anterior and posterior segment examination. Participants with a best-corrected visual acuity of 20/20 according to Snellen chart, an intraocular pressure of 10–20 mmHg on Goldmann applanation tonometry, and a spherical equivalent refraction within a range of ±2.00 dioptres were included in the study. Patients with severe myopia and hypermetropia, amblyopia, glaucoma, retinal vascular disease (such as diabetic and hypertensive retinopathy), or media opacity (such as corneal scar, cataract, and intravitreal haemorrhage) affecting OCT measurements were excluded from the study.
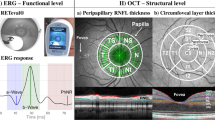
A spectral-domain optical coherence tomography (SD-OCT) (Heidelberg Engineering Inc., Heidelberg, Germany) was used for the OCT measurements. All measurements were performed by an experienced technician. The macular retinal layers were analysed by automatically segmenting them into the RNFL, GCL, and IPL using the SD-OCT software in the retina mode (Fig. 1). Macular scanning was performed as three concentric circles with 1 mm (inner), 3 mm (intermediate), and 6 mm (outer) diameters centred at the fovea, according to the Early Treatment Diabetic Retinopathy Study Protocol. The internal (parafoveal) and external (perifoveal) circles, except for the fovea-centred circle, were evaluated in four quadrants (superior, inferior, nasal, and temporal). In total, the macular scans were evaluated in nine quadrants (Fig. 2).
Statistical analysis
The effect size was estimated based on a previous publication that has a similar methodology as 0.60 (Cohen d) [17]. To find significant difference between any of the bipolar groups and control group in terms of central quadrant measurements, minimum required sample size was determined as 46 for each group (α = 0.05, 1−β = 0.80). G power version 3.1.9 was used for power analysis.
The Statistical Package for Social Science software version 18.0 was used to analyse the data. Categorical variables were expressed as number and percentage, and continuous variables were expressed as mean and standard deviation (SD). The Chi-square test was used to compare categorical variables. The Kolmogorov–Smirnov test was used to confirm a normal distribution of the variables. Analysis of variance was used to compare normally distributed continuous variables between four groups, whereas the Kruskal–Wallis test was used to compare non-normally distributed continuous variables between four groups. The Student’s t test was used to compare normally distributed continuous variables between two groups, whereas the Mann–Whitney U test was used to compare non-normally distributed continuous variables between two groups. When both variables were normally distributed, the correlation coefficients and their statistical significance were calculated with the Pearson correlation test. When at least one variable was not normally distributed, the correlation coefficients and their statistical significance were calculated with the Spearman correlation test. A p value ≤ 0.05 was considered statistically significant.
Results
Each group consisted of 25 male and 25 female patients. The mean age (p = 0.242) and gender distribution (p = 1) of the patient and control groups are given in Table 1. No statistically significant difference was noted in the mean age and gender distribution between these groups.
No statistically significant difference was found between the patient groups in terms of disease duration (DD) (p = 0.633), whereas a statistically significant difference was detected between the patient groups in terms of the number of hospitalizations (NH) (p = 0.004), the number of manic episodes (NME) (p = 0.019), the number of depressive episodes (NDE) (p < 0.001), the HDRS scores (p < 0.001), and the YMRS scores (p < 0.001) (Table 1) In addition, all of the patients were treated with psychotropic drugs. In the euthymic group 35 patients were treated with mood stabilizers (DDD) plus antipsychotics (AP), three patients were treated with AP only and 12 patients were treated with DDD only. In the manic group 45 patients were treated with DDD plus AP, five patients were treated with AP only. In the depressive group 40 patients were treated with DDD plus AP, eight patients were treated with DDD plus AP plus antidepressant, two patients were treated with AP only.
Comparison of the GCC thicknesses between the patient and control groups revealed a significantly greater mean thickness of perifoveal superior quadrant in the euthymic, manic, and depressive patient groups than in the control group (p < 0.001) and a significantly greater mean thickness of the perifoveal inferior quadrant in the manic and euthymic patient groups than in the control group (p = 0.002). No statistically significant difference was noted for the mean thicknesses of the perifoveal superior and inferior quadrants between the three patient groups. No statistically significant difference was found in the other quadrants between the patient and control groups. The comparison of GCC thicknesses by quadrants in the patient and control groups is shown in Table 2.
A significant negative relationship was found between the mean thicknesses of the parafoveal superior (r = −0.225, p = 0.006) and temporal (r = −0.220, p = 0.007) quadrants and the NH, whereas a significant positive relationship was noted between the mean thicknesses of the parafoveal superior quadrant (r = 0.352, p = 0.012) and the perifoveal superior (r = 0.390, p = 0.005) and inferior (r = 0.288, p = 0.043) quadrants and YMRS scores in the patients with manic BD. The relationships between the clinical characteristics of patients and the GCC quadrants are given in Table 3.
Discussion
Many genetic, biological, and neuroimaging studies have been conducted to explain the pathophysiology of BD, but the findings are largely inconsistent due to the heterogeneity of this disease. An increasing number of studies have therefore been designed to use neuroimaging methods to reveal the neuronal damage and etiopathogenesis associated with BD.14Some studies have shown signs of neuronal degeneration in patients with first-episode BD, whereas other studies have linked these findings to the longitudinal course of BD and the severity of the mood episodes. Whether these degeneration signs represent a state or trait markers remains in question [14, 20, 21].
To our knowledge, our study is the first study to compare OCT measurements in patients with BD with different mood episodes. For example, the studies of Kalenderoglu et al. [15], Polo et al. [17], and Garcia et al. [12] included only euthymic bipolar patients, while the studies of Mehraban et al. [16] and Khalil et al. [13, 14] included bipolar patients with any mood episode (euthymic, manic, or depressive). Kalenderoglu et al. [15] reported that the RNFL thickness was decreased in all sectors in patients with euthymic BD, but this decrease was not significant when compared to the control group. They also found a significantly lower GCL volume in the patient group than in the control group. In the present study, the finding that the decrease in the GCL volume was much more pronounced than the decrease in the RNFL thickness could reflect that neurodegeneration begins from the GCL (which displays neuronal cell bodies) and then gradually progresses to axonal degeneration.
Garcia et al. [12] evaluated the GCL, IPL, and INL thicknesses separately in patients with euthymic BD and found a significant reduction in the GCL and IPL thicknesses in some sectors. However, they revealed that the INL thickness was significantly higher in most sectors. Our study demonstrated that GCC thicknesses were higher in almost all quadrants in three patient groups (euthymic, manic, and depressive) compared to the control group and that the results were significant in two quadrants (perifoveal superior and inferior) (Table 2).
No other study in the literature has shown this increase in retinal thickness in patients with an acute episode of BD. However, Ascaso et al. [22] compared schizophrenia patients with and without a recent illness episode (RIE) with healthy controls and found a decrease in the RNFL thickness in schizophrenia patients without a RIE (who had no psychotic symptoms and had been stable for the past 6 months). They explained these findings as possibly indicating the occurrence of neuroinflammation during disease episodes; this inflammation would increase the retinal thickness, thereby masking the decrease in the RNFL thickness. Another study showed that the RNFL thickness increased in patients with an acute exacerbation of multiple sclerosis and this increase was associated with neuroinflammation [23]. Inflammatory and immunologic changes have been identified as mechanisms that play a role in the pathophysiology of BD [6,7,8]. In light of this information, inflammatory processes are thought to play a role in the increased retinal thickness in patients with manic and depressive episodes of BD. Further studies evaluating patients with an acute episode of BD should be conducted to confirm these changes in the retina and to explain why the GCC thickens in patients with BD.
Comparison of our study results with those of published OCT studies conducted on patients with euthymic BD, revealed unexpectedly higher GCC thicknesses in our euthymic patients. Our study included patients who were euthymic for at least 6 months, suggesting that the inflammatory process lasts longer than 6 months or continues basally. To the best of our knowledge, the previous OCT studies on patients with euthymic BD did not provide information on the length of time these patients had been euthymic. Future studies that concomitantly evaluate inflammatory markers and OCT measurements may be useful in confirming these results.
In our study, the relationship between clinical features indicating disease severity (DD, NH, NME, NDE, and scale scores) and the GCC thicknesses was investigated to test whether changes in these features were associated with disease severity. Some studies in the literature have examined the relationship between clinical features and OCT parameters, but the results have been inconsistent. For example, Mehraban et al. [16] reported a significant relationship only between disease duration and RNFL thickness loss, whereas Khalil et al. [14] determined a positive relationship only between disease duration and the inferior quadrant RNFL thickness. Kalenderoglu et al. [15] showed a significant relationship between the GCL and CGI, YMRS, disease duration, and hospital stay in patients with euthymic BD. They also emphasized that the presence of a significant relationship between GCL volume reduction and disease duration indicated progressive degeneration. However, examining the relationship between the decrease in the RNFL and GCL thicknesses and the scale scores (such as CGI and YMRS) that indicate clinical severity in patients with euthymic BD seems contradictory. Therefore, the relationship between the GCC and DD, NH, NME, NDE was evaluated in all patients in our study. Our study results showed no significant relationship between the GCC and DD, NME, NDE, HDRS scores, but a significant negative relationship was detected between the GCC and NH in some quadrants. In addition, GCC and YMRS scores showed a statistically significant positive relationship that could be explained by inflammation study, but the relationship between the GCC and YMRS scores in the patients with manic BD and between the GCC and HDRS scores in the patients with depressive BD were also examined severity.
Our study has several strengths. One strength was that it had the largest number of cases to date for examining retinal changes in patients diagnosed with BD. A second strength is that it evaluated manic and depressive bipolar patients, as well as euthymic patients. However, our study also has some limitations. The most important limitation is that our study had a cross-sectional design, as performing OCT measurements at regular intervals from the early stages of BD could have yielded more satisfactory results regarding the progressive course of BD. The other major limitation is the possible effects of psychotropic drugs on the OCT measurements. This possibility has not been studied in detail before, and all patients included in the study were taking antipsychotics and/or mood stabilizers (lithium-valproic acid) [24]. The potential neuroprotective effects of atypical antipsychotics cannot be excluded, and this could have affected the study results [25].
Both lithium and valproic acid are thought to have neuroprotective effects, so these drugs could lead to different changes in the brain volume [26]. Lithium has been shown to increase grey matter volume in different parts of the brain in patients diagnosed with BD [27]. However, another study revealed that the use of valproic acid was associated with decreased brain volume in epilepsy patients [28]. Kalenderoglu et al [15]. demonstrated a thinner RNFL thickness in valproic acid users than inpatients that did not use valproic acid. In addition, some of the patients included in our study were taking antidepressants, and previous studies have shown that patients with depression had some differences in retinal parameters (measured electrophysiologically) but antidepressant treatment normalized these findings [29, 30]. Our study was not designed to detect differences in OCT measurements between patients receiving antipsychotics, mood stabilizers, antidepressants or other medications; therefore, many potential drug effects could have affected these results and should be investigated in future studies. Another limitation is that inflammatory markers, such as cytokines, and acute phase reactants were not studied in the patient and control groups. Concomitant evaluation of these inflammatory markers and OCT measurements could serve as a second indicator of inflammation in patients diagnosed with BD, and their correlation with retinal thickness changes detected by OCT could increase the reliability of OCT.
Conclusion
In our study, SD-OCT was used to evaluate GCC thickness changes in patients with euthymic, manic, and depressive BD, and to determine the relationship between the clinical features of patients and these changes. No significant difference was found among the three patient groups in terms of the GCC thickness. Moreover, GCC thickness was higher in almost all quadrants in three patient groups than in the control group, but the difference was significant in two quadrants. These results can be explained by inflammation, which therefore has a possible role in the pathophysiology of BD. Nevertheless, the best approach for determining whether this increase in the GCC thickness is a state or a trait marker would be to study different mood episodes in these patients using various psychometric tools that indicate disease severity.
Summary
What was known before
-
Few studies in the literature have examined the retinas of the patients with BD irrespective of episodes of the disease.
-
No study has yet examined the retina during manic and depressive episodes in BD patients.
What this study adds
-
No significant difference was found among the three episodes of BD patient groups in terms of the GCC thickness.
-
GCC thickness was higher in almost all quadrants in BD patient groups than in the control group.
References
Akiskal HS, Bourgeois ML, Angst J, Post R, Moller H, Hirschfeld R. Re-evaluating the prevalence of and diagnostic composition within the broad clinical spectrum of bipolar disorders. J Affect Disord. 2000;59(Suppl 1):S5–S30.
Saglam Aykut D, Civil Arslan F, Ozkorumak Karaguzel E, Aral G, Karakullukcu S. The relationship between neutrophil-lymphocyte, platelet-lymphocyte ratio and cognitive functions in bipolar disorder. Nord J Psychiatry. 2018;72(2):119–23.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association: Washington, DC; 2000 [text revision].
Phillips ML, Vieta E. Identifying functional neuroimaging biomarkers of bipolar disorder: toward DSM-V. Schizophr Bull. 2007;33(4):893–904.
Karadağ AS, Kalenderoğlu A. Psychiatric disorders and eye: optical coherent tomography in psychiatry aspect. Turkish J Clin Psychiatry. 2017;20(3):27–237.
Kalelioglu T, Akkus M, Karamustafalioglu N, Genc A, Genc ES, Cansiz A, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as inflammation markers for bipolar disorder. Psychiatry Res. 2015;228(3):925–7.
Mert DG, Terzi H. Mean platelet volume in bipolar disorder: the search for an ideal biomarker. Neuropsychiatr Dis Treat. 2016;12:2057–62.
Rosenblat JD, Brietzke E, Mansur RB, Maruschak NA, Lee Y, McIntyre RS. Inflammation as a neurobiological substrate of cognitive impairment in bipolar disorder: evidence, pathophysiology and treatment implications. J Affect Disord. 2015;188:149–59.
Bock M, Paul F, Dörr J. Diagnostik und Verlaufsbeurteilung der Multiplen Sklerose: Stellenwert der optischen Kohärenztomographie. Nervenarzt. 2013;84(4):483–92. https://doi.org/10.1007/s00115-012-3707-2. German
Jindahra P, Hedges TR, Mendoza-Santiesteban CE, Plant GT. Optical coherence tomography of the retina: applications in neurology. Curr Opin Neurol. 2010;23(1):16–2.
Jeoung JW, Choi YJ, Park KH, Kim DM. Macular ganglion cell imaging study: glaucoma diagnostic accuracy of spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(7):4422–9.
Garcia-Martin E, Gavin A, Garcia-Campayo J, Vilades E, Orduna E, Polo V, et al. Visual function and retinal changes in patients with bipolar disorder. Retina. 2019;39(10):2012–21.
Khalil DH, Said MM. Peripapillary retinal nerve fiber layer and ganglion cell complex degeneration in Egyptian patients with bipolar disorder. Eye. 2019;33(12):1852–8.
Khalil MA, Saleh AA, Gohar SM, Khalil DH, Said M. Optical coherence tomography findings in patients with bipolar disorder. J Affect Disord. 2017;218:115–22.
Kalenderoglu A, Sevgi-Karadag A, Celik M, Egilmez OB, Han-Almis B, Ozen ME. Can the retinal ganglion cell layer (GCL) volume be a new marker to detect neurodegeneration in bipolar disorder? Compr Psychiatry. 2016;67:66–72.
Mehraban A, Samimi SM, Entezari M, Seifi MH, Nazari M, Yaseri M. Peripapillary retinal nerve fiber layer thickness in bipolar disorder. Graefes Arch Clin Exp Ophthalmol. 2016;254(2):365–71.
Polo V, Satue M, Gavin A, Vilades E, Orduna E, Cipres M, et al. Ability of swept source OCT to detect retinal changes in patients with bipolar disorder. Eye. 2019;33(4):549–56.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56.
Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429–35.
Frey BN, Zunta-Soares GB, Caetano SC, Nicoletti MA, Hatch JP, Brambilla P, et al. Illness duration and total brain gray matter in bipolar disorder: evidence for neurodegeneration? Eur Neuropsychopharmacol. 2008;18(10):717–22.
Vita A, De Peri L, Sacchetti E. Gray matter, white matter, brain, and intracranial volumes in first-episode bipolar disorder: a meta-analysis of magnetic resonance imaging studies. Bipolar Disord. 2009;11(8):807–14.
Ascaso FJ, Rodriguez-Jimenez R, Cabezon L, Lopez-Anton R, Santabarbara J, De la Camara C, et al. Retinal nerve fiber layer and macular thickness in patients with schizophrenia: influence of recent illness episodes. Psychiatry Res. 2015;229(1-2):230–6.
Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133(Pt 6):1591–601.
Yildiz M, Alim S, Batmaz S, Demir S, Songur E, Ortak H, et al. Duration of the depressive episode is correlated with ganglion cell inner plexifrom layer and nasal retinal fiber layer thicknesses: optical coherence tomography findings in major depression. Psychiatry Res Neuroimaging. 2016;251:60–66.
Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009;39(11):1763–77.
Chiu CT, Wang Z, Hunsberger JG, Chuang DM. Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharm Rev. 2013;65(1):105–42.
Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65(9):1017–32.
Pardoe HR, Berg AT, Jackson GD. Sodium valproate use is associated with reduced parietal lobe thickness and brain volume. Neurology. 2013;80(20):1895–1900.
Bubl E, Ebert D, Kern E, van Elst LT, Bach M. Effect of antidepressive therapy on retinal contrast processing in depressive disorder. Br J Psychiatry. 2012;201:151–8.
Bubl E, Kern E, Ebert D, Bach M, Tebartz van Elst L. Seeing gray when feeling blue? Depression can be measured in the eye of the diseased. Biol Psychiatry. 2010;68(2):205–8.
Author information
Authors and Affiliations
Contributions
YC and EM designed the study and the computational framework and analysed the data. AMC, II, IEC, MO, and IE contributed to the interpretation of the results and revising work critically. YC wrote the manuscript with input from all authors. YC and EM conceived the study and were in charge of overall direction and planning.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cokunlu, Y., Mirza, E., Caliskan, A.M. et al. Ganglion cell complex thickness changes in patients with different states of bipolar disorder. Eye 36, 1034–1041 (2022). https://doi.org/10.1038/s41433-021-01580-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01580-4