Abstract
Purpose
To evaluate the use of optical coherence tomography angiography (OCTA), structural OCT and fundus fluorescein angiography (FFA) to distinguish neovascularisation elsewhere (NVE) from intra retinal microvascular abnormalities (IRMA) and their use in early detection and possible risk assessment for vitreous haemorrhage.
Methods
A cross-sectional study of a consecutive series of patients with suspected NVE and IRMA using clinical examination and FFA, were examined further with OCT and OCTA. Treated and untreated eyes were also compared.
Results
Images from 33 eyes of 26 patients, showed 27 NVE and 14 IRMA lesions based on clinical examination +/− FFA. Lesions were re-classified as NVE in 22 eyes. Ten eyes had received past treatment. In all 10 treated eyes, vascular flow and vitreous connection were found but not FFA leakage. In 18/22 eyes with NVE there was a breach of the internal limiting membrane (ILM), in 4 eyes there was FFA leak, ILM outpouching but no breach. In two eyes, NVE originated from sea fan IRMA. Ten eyes images were classified as IRMA only with no FFA leak, or ILM breach. The relation of pre-retinal NVE to the vitreous can be visualised.
Conclusion
Lesions, considered to be NVE, after further assessment with OCT and OCTA, can be intra-retinal, with ILM disruption but no ILM breach and leakage on FFA. ILM disruption maybe one of the earliest signs of the development of neovascularisation. Visualisation of the relation to the posterior vitreous is likely to be useful in assessing risk of vitreous haemorrhage.
Similar content being viewed by others
Introduction
Diabetic retinopathy is a leading cause of blindness in working-age populations [1]. Proliferative diabetic retinopathy (PDR) affects 9% of patients with diabetic retinopathy, which untreated may lead to vitreous haemorrhage and fibrosis progressing to tractional retinal detachment, resulting in severe visual impairment.
The detection and treatment of PDR is therefore an important part of managing diabetic retinopathy.
Historical descriptions of retinal neovascularisation were based on histopathologic definitions. Intra-retinal microvascular abnormalities (IRMA) were defined as tortuous intra-retinal segments and are indicative of severe non-proliferative diabetic retinopathy. The distinction between IRMA and neovascularisation elsewhere (NVE) in the Early Treatment of Diabetic Retinopathy (ETDRS) and Diabetic Retinopathy Study (DRS) utilised colour stereoscopic photographs, defining NVE as being internal to the retina [2,3,4]. Fluorescein angiography (FA) leakage was reported as occurring in both NVE and IRMA lesions in the ETDRS study therefore, the presence of fluorescein leakage was not a differentiating feature between proliferative and non-proliferative diabetic retinopathy in landmark clinical trials [3]. However, in clinical practice, the presence of fluorescein leakage is often used to diagnose PDR. Structural optical coherence tomography (OCT) new vessels are defined by internal limiting membrane (ILM) breach [5]. Optical coherence tomography angiography (OCTA) imaging enables a detailed visualisation of retinal vascular structure.
In this study, vascular lesions clinically diagnosed as IRMA or NVE using colour and red-free photographs as part of the patients’ standard care were studied with OCT, OCTA and FFA. The aims were to determine if morphological, structural and vitreous related factors could be identified in different imaging modalities to distinguish IRMA from NVE.
Methods
This was a retrospective observational cohort study, reviewing patients seen at the Ophthalmology department in the Royal Victoria Infirmary, Newcastle, United Kingdom. The research and development office at the Newcastle upon Tyne Hospitals Foundation Health Trust approved this study. This study followed the principles of the Declaration of Helsinki. Informed consent was obtained for all patients included in this study.
A consecutive series of eyes from adult diabetic patients attending the medical retina clinic clinically diagnosed with IRMA or NVE were included and imaged with wide-field colour photography, (Optos plc, Dunfermline, Scotland, UK), structural OCT and OCTA (Heidelberg Spectralis optical coherence tomography angiography, Heidelberg, Germany). Both treatment naïve and previously treated eyes were included. Fundus photography, structural OCT and OCTA were performed on the same day. FFA was performed as determined by the diagnosing clinician if indicated and performed within two weeks. A 3 mm cube for OCTA scan was performed over the area of interest. This included structural OCT and OCTA images. A series of OCT section images were acquired to create the cube of data.
The high-resolution scan (5.7 μm/pixel) facilitates visualisation of the retinal capillaries. In the user interface, two types of section images can be obtained. One image has the same orientation as the original B-scans and the other image corresponds to a section orthogonal to the original B scan. Structural OCT images and corresponding blood flow information are combined into a fusion image. This provides direct visual correlation of structure and flow information. Relative transparency of the superimposed blood flow and structural information may be adjusted via a slider in the user interface.
Patients were excluded with concurrent retinal disease, corneal pathology or media opacities precluding clear imaging.
The following were assessed:
-
(1)
Presence or absence of fluorescein leakage if performed.
-
(2)
OCT determined NVE, defined as ILM breach.
-
(3)
ILM out pouching in intra-retinal lesions.
-
(4)
Retinal layer of origin of the lesion.
-
(5)
Area of the vascular lesion (NVE or IRMA) measured in the enface OCTA image (mm2) with Heidelberg software measuring tool.
-
(6)
Size of ILM breach measured in the OCT/ OCTA fusion image with the Heidelberg built in software measuring tool. This was measured at the level of the ILM by first selecting the measure tool from the menu, then placing the cursor at one end of the ILM breach, then moving the cursor to the other end of the ILM breach. The size of the area measured was automatically displayed and recorded.
-
(7)
If a lesion had breached the ILM whether the lesion was connected to the vitreous. The vitreous was classified as attached if the posterior hyaloid face was intact, detached if not intact, and tethered if the NVE complex was attached to the posterior hyaloid face. The Spectralis OCTA pre-defined slabs do not include a specific vitreo-retinal interface slab. No specific imaging technique was therefore used to image the posterior hyaloid face in this study. The posterior hyaloid face was visualised on all images included in this study.
-
(8)
The degree of vascularity of NVE complex. This was described as vascular if OCTA imaging depicted the majority of the lesion as yellow indicating vascular flow, fibrovascular if imaging illustrated a mixture of yellow and white, suggesting early fibrosis, and fibrous if imaging demonstrated the NVE complex as white. Yellow and white colour codes represent a qualitative rather than quantitative measure of the degree of vascularity of the NVE complex in Spectralis OCTA imaging. A yellow-white gradient index (Word, Microsoft Office 2016, Redmond, Washington, USA) was used to provide a qualitative measure of degree of vascularity.
-
(9)
The pattern of the NVE complex. These were graded as flat (Diagram 1), if no projection into the vitreous was present, tabletop (Diagram 2), if pegs of hyper-reflective tissue were linked to flat complexes, and forward (Diagram 3), if there was a significant projection into the vitreous. Exuberant vascular proliferation (Diagram 4) was defined as large NVE complexes containing multiple vascular loops. Images were anonymized and were evaluated independently on two consecutive occasions by a trained retinal specialist (JD). NVE lesions were further categorised as having received past treatment or treatment naïve. A clinical judgement was made on whether the lesion was active and so the eye needed more treatment. A chi-square test was used to analyse differences between these two groups. Results were analysed between eyes rather than lesions as treatment effects would confound analysis of individual vascular lesions occurring in the same eye. Segmentation was performed with built in software to delineate the inner limiting membrane (ILM) to the inner nuclear layer (INL). Manual adjustment was performed if the retinal layers were extensively disrupted due to underlying pathology so confounding default slab definitions. The projection artefact removal tool was used to remove replication of more superficial vessels in posterior layers.
Results
OCTA images of 41 eyes of 33 patients attending the medical retina clinic were acquired. Nine OCTA images were excluded because of poor quality. Therefore, 33 eyes of 26 patients were included. Mean age was 45 years (range 22–71). Twenty patients were male and six female. Twenty-three eyes were treatment naïve and 22 eyes were re-classified as having NVE. Ten eyes had received past treatment (9 past retinal laser treatment and 1 patient past anti-vascular endothelial growth factor injection). A total of 41 lesions were examined comprising 14 IRMA and 27 NVE lesions. Some eyes included more than one vascular lesion and 10 eyes contained only lesions classified as IRMA. Data related to NVE, vitreous morphology and layer of origin are presented in Table 1.
NVE
Clinically evaluated active NVE, judged to require treatment, were found in15/22 eyes. These eyes demonstrated pre-retinal hyper-reflective NVE complexes. Six eyes were noted to have flat complexes incorporated within the inner retinal layers extending through the ILM and attached to the outer aspect of the posterior hyaloid face. Five eyes had tabletop complexes consisting of pegs of hyperreflective tissue extending across the ILM and forming a flat tabletop configuration attached to the posterior hyaloid face but not disrupting the vitreous. Eleven eyes demonstrated elevated (described as forward in Table 1) NVE, with elevated pegs of hyper-reflective tissue extending across the posterior hyaloid surface with disruption into the vitreous body. In total there were 27 NVE lesions and 23/27 originated from the surface of a large vein. NVE originating on the surface of a large vein could remain small without multiple branches before breaching the ILM. If leak on FFA is used to define NVE there were 4 lesions, which were intra-retinal NVE as they leaked on FFA and had outpouching and disruption of the ILM but had not breached the ILM. (Fig. 1 and supplementary information). Two of these eyes were noted to have developed NVE arising from sea-fan IRMA lesions. These leaked on FFA, had ILM out pouching, but had not breached the ILM. Both of these eyes had had prior retinal laser treatment, which may have introduced a confounding effect on the interpretation of the origin of the lesion (Fig. 2).
From left to right: A Enface OCTA image of NVE lesion outlined in blue; B OCT/ OCT fusion image, section along B scan direction, represented by green line; there is out pouching of the ILM and ILM disruption but no breach; C FFA image demonstrating leakage of right inferotemporal NVE lesion. A colour key provides an index for determining intensity of the yellow and white areas.
From left to right: A Enface OCTA image demonstrating seafan configuration; B OCT/ OCTA fusion image, OCTA data represented by yellow overlay, section along B scan direction, represented by green line, very small multiple ILM breaches evident; C Section is along scanning axis, orthogonal to B scan direction, represented by blue line, illustrating vitreous dots and the appearance of both ILM outpouching and multiple small ILM breaches. A colour key provides an index for determining intensity of the yellow and white areas.
IRMA
Ten eyes were classified as having IRMA alone and in total there were 14 IRMA lesions. Three out of 14 IRMA lesions originated from the surface of a large vein these lesions were associated with areas of capillary closure visible on OCTA and FFA imaging and structural OCT in these cases showed ILM outpouching but no ILM disruption or breach, no vitreous dots and no fluorescein leakage was present (Fig. 3).
From left to right: A Enface OCTA image of IRMA lesion associated with capillary drop out; B OCT/OCTA fusion image, OCTA data represented by yellow overlay, IRMA not exhibiting ILM breach, or vitreous dots ILM outpouching present but no ILM disruption; C FFA demonstrates no fluorescein leakage in area indicated by white circle. A colour key provides an index for determining intensity of the yellow and white areas.
Regression of NVE
In seven eyes with imaging of NVE clinically evaluated to be inactive after treatment there was still flow detectable on OCTA although it was reduced (figures shown in Supplementary information). In one case there was active NVE with OCTA detected flow and FFA leak but another lesion with OCTA detected flow in front of the ILM. with no leak on FFA. As the NVE complexes were generally attached to an elevated posterior hyaloid it is likely there was still a risk of bleeding. Flow metrics are not currently available with Heidelberg imaging, so interpretation is subjective.
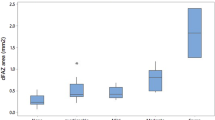
Size of ILM breach and relation to area of vascular lesion
We found no association between the size of the ILM breach and the area of the NVE lesion. There was a large variation in the size of the ILM breach (Fig. 4).
Regression analysis did not show the size of ILM breach predicted the size of the NVE lesion (p = 0.36). The box plot above the graph shows the variability in the measurement of ILM breach. The box plot to the right of the graph shows variability in the measurement of the area of the NVE lesion. The bottom of the box represents the 25th percentile, the top of the box represents the 75th percentile and the line in the middle represents the 50th percentile. The whiskers represent the highest and lowest values that are not outliers or extreme values. The asterisk beyond the whiskers represents an outlier or extreme value.
Discussion
Our study demonstrates that some vascular lesions, can be intra-retinal and leak on FFA or have grown through the ILM, not leak but still have vascular flow. We found lesions with ILM outpouching and ILM disruption but no ILM breach that had FFA leak. These lesions may represent early intra-retinal NVE. In some cases, the intra-retinal network can be large before the ILM is breached and in others very small. We found no association between size of ILM breach and area of the NVE lesion. Small lesions that break through the ILM occurred on large vessels. With small lesions FFA can help draw attention to the area of neovascularisation by the presence of leakage but could be also missed on FFA due to overlap with the vein. Cross-sectional structural OCT is required to clarify ILM breach and show connection to the vitreous. Structural OCT is therefore the most sensitive method for detecting NVE that are at risk of leading to vitreous haemorrhage. Diabetic retinal neovascularisation results from vascular proliferation from the venous side of the circulation culminating in breach of the internal limiting membrane [6]. In the early stages of neovascularisation new vessels remain parallel and flat with regard to the retinal surface. As retinal neovascular tissue matures, fronds develop, and cartwheel-like shapes form [7].
Clinically, IRMA tend to be larger calibre vessels than retinal new vessels and are considered to represent shunt vessels bypassing the retinal capillary bed. In contrast, neovascularisation appears clinically as fine vascular loops or networks on the surface of the retina extending into the vitreous. Clinically differentiating between new vessels and IRMAs may be challenging particularly if IRMAs are widespread, or if new vessels do not show characteristic features [8]. It is has long been debated whether IRMA are pre-cursors for NVE that grow through the ILM.
Grading of diabetic retinopathy in the DRS and the ETDRS studies was based on assessment of stereoscopic colour photographs [3, 4]. IRMA were defined as tortuous intra-retinal vascular segments varying in calibre from barely visible to 31 μm (approximately one forth the width of a major vein at the disc margin). New vessels were defined as being in front of the retina and graded according to the area involved < or > ½ disc area.
Textbooks often state FFA can distinguish between these lesions by defining new vessels as vascular outgrowths that leak and IRMA lesions as not leaking [9].
Histo-pathologically, NVE are defined as lesions breaching the ILM with extension into the posterior hyaloid, IRMA do not exhibit the same features [10]. Lee et al. examined the SD-OCT features differentiating IRMA from NVE lesions in a small-scale retrospective study. IRMA was postulated to occur in two stages. Stage 1 was defined as hyper-reflective dots in the inner retina indicative of inflammatory changes without ILM outpouching and stage 2 was defined as ILM outpouching. Stage 1 NVE was defined as ILM breach without growth along the posterior hyaloid [5]. We suggest areas of ILM outpouching with a smooth contour are more likely to represent IRMA. Lesions that disrupt the ILM from a smooth contour to a mushroom-like configuration may be indicative of ILM disruption, which may represent small ILM breaches. These lesions although small may be at high risk of progression to grow through the posterior hyaloid and form vitreous connections.
In a cross-sectional retrospective analysis of 16 eyes of patients with PDR using spectral-domain OCT, IRMA were defined as lesions, which do not project into the vitreous, with an attached posterior hyaloid, and no fluorescein leakage. The majority of lesions were intra-retinal; however, there were focal areas where IRMA appeared to protrude through the ILM but not into the overlying vitreous [8]. NVE vessels projected through the attached posterior hyaloid into the vitreous and fluorescein angiography demonstrated leakage. NVE were seen as hyper-reflective loops of relatively homogenous hyper-reflectivity. The study mirrors our findings of overlap between features of IRMA and NVE. We suggest that the reported cases where the lesion projected through the ILM were early NVE. In cases where there has been some treatment there may not be FFA leak.
Descriptions of IRMA have been provided by examination of autopsied specimens [10].
The distinctive morphology of IRMAs was the occurrence of multiple microvascular lumina surrounded by a perivascular cuff, located close to the inner limiting membrane with occasional endothelial fenestrae in vessels located within the neurosensory retina. Blending of some of these intra-retinal, microvascular IRMA like lesions into pre-retinal, neovascular proliferations was also apparent. These lesions appeared histologically distinct from collaterals associated with retinal vein occlusion. IRMA structures were observed with a cuff comprised of collagen fibrils, resembling the extracellular matrix of pre-retinal neovascular membranes [10].
Endothelial fenestrations were noted occasionally in IRMA [10]. Fenestrations represent morphologic similarity to new vessel proliferation and may explain why IRMAs are sometimes associated with fluorescein leakage. Morphologic similarity and close spatial correlation are suggestive of the neovascular potential of IRMAs [10]. Chang described a series of 57 neovascular lesions in diabetic patients followed before the development of new vessels [11]. Seventy percent of new vessels were found to arise near pre-existing IRMAs; only 30% could not be traced to IRMAs. This suggests that only some IRMAs may progress to neovascularisation. A proportion of clinically defined IRMA lesions may degenerate over time due to a lack of vaso-proliferative stimuli [11].
Structural OCT may be utilised to identify neovascularisation above the internal limiting membrane but is unable to distinguish vascular components from associated fibrous tissue structures. Hence, OCT is limited to defining the localization of neovascularisation. OCTA can show vascularisation both above and below the ILM.
Defining NVE as being in front of the ILM, de Carlo et. al. described a method for detection of pre-retinal neovascularisation using OCTA and also described the findings in the surrounding vasculature [12]. In a series of 52 eyes, 92% of the pre-retinal neovascularisation was noted to border an area of capillary non-perfusion.
IRMA were directly adjacent to the pre-retinal neovascularization in 50% of eyes. This supports the observation that some IRMA lesions represent portions of pre-retinal neovascularisation that have not yet broken through the ILM. This is in agreement with our results suggesting that IRMA and NVE represent a continuum rather than distinct clinical entities. Pan et. al. attempted to classify NVE into three patterns based on the retinal layer of origin of the vessels and location in the vicinity of a major vessel or in association with IRMA [13]. Type 1 was described as originating from the ganglion cell layer, type 2 from the inner nuclear layer and type 3 from sea fan IRMA [13]. In our study, we found 16/22 (73%) NVE were classified as type 1, 4/22 (18%) as type 2 and 2/22 (9%) as type 3. Compared to Pan et al. our result show more type 1 lesions; however, we did not include NVD, which may account for this difference. IRMA were described as breaching the ILM in a few cases and NVE were defined as breaching the ILM and displaying leakage on FFA [13]. The clinical relevance of retinal layer of origin remains to be determined. Limitations of the study by Pan et al. were projection artefacts and difficulty distinguishing arteries from veins on OCTA. Correlation with other imaging modalities such as FFA may help differentiate arteries from veins, however, this may become increasingly difficult with smaller vessels. The Spectralis system we used attempts to remove projection artefact however, the reliability of retinal layer of origin measurements remains uncertain. This is because of an oversimplification of the three-dimensional retinal circulation by current OCT imaging techniques. Images are captured at a specific time point and the vessel pattern may change as the NVE structure evolves. NVE patterns may be intrinsically related to the vitreous rather than inherently different types of NVE growth being present.
By examining SD OCT and FFA leak Muqit et al. concluded that by looking at the regression pattern of vessels after pan-retinal photocoagulation in relation to location to the internal limiting membrane NVE could be intra-retinal as well as have grown through the ILM [14]. Chatziralli et al. evaluated regression of NVE after pan-retinal photocoagulation based on its location relative to the ILM in a retrospective case series [15]. The below ILM group was associated with a twofold enhanced regression of NVE in comparison to the above ILM group [15].
In 2/22 (9% eyes of our series), NVE appeared to arise from IRMA. Lee et al. described three of ten IRMAs progressing to pre-retinal neovascularisation in their study [5]. This agrees with our finding that IRMA may represent segments of pre-retinal neovascularisation that have yet to breach the ILM. Recently, Russell et al. showed in a longitudinal prospective observational series of two treatment naïve PDR patients that NVE could develop from IRMA after three months despite treatment with 360-degree pan-retinal photocoagulation laser treatment [16]. The details of the laser treatment administered such as spot size and number of burns was not reported and the results are limited by the small size and short duration of the study.
OCTA may allow assessment of the regression, measured by size or flow, of pre-retinal neovascularisation following therapeutic interventions such as laser or anti-VEGF therapy. A study has shown a decrease in neovascularisation area following pan-retinal photocoagulation using OCTA [17]. However, OCTA does not permit an assessment of leakage from retinal neovascular lesions. In our study, treated patients still had some signs of vascular flow if NVE were located above the ILM.
This is consistent with Ishibazawa et al. who showed a lower regression rate compared to intra-retinal NVE, suggesting earlier treatment could be more effective, especially before NVE has attached to the vitreous [17].
OCTA uses laser reflectance of moving red blood cells to depict vessels through different segmented layers. Movement of a NVE frond or the attached vitreous may generate an artefactual flow signal. Flow cannot currently be quantified from images. The combination of more or less flow based on signal brightness and the nature of the vitreous attachment provides some information on the risk of vitreous haemorrhage. These parameters maybe more accurate than clinical assessment, which involves evaluating the size of the NVE, the presence of haemorrhage and judging whether NVE is elevated and attached to the vitreous. Evaluation of these factors may be easier through visualisation of an OCT image.
The small field of view available with the current Heidelberg OCTA system limits its use to specific areas of concern, rather than adoption as practical screening tool for detection of NVE and IRMA. This may have introduced a source of selection bias in our results together with exclusion of poor-quality imaging. Other wide field OCT systems exist and are in development. Higher resolution and three-dimensional imaging capability may allow a better understanding of the underlying pathological processes.
Our study suggests that the optimal assessment for diabetic neovascularisation is a combination of OCT, OCTA and FFA imaging. This is likely to detect small early new vessels before they have grown through the ILM into the vitreous. Imaging could be utilised for close monitoring. Potentially earlier treatment could be administered before NVE attachments to the vitreous develop, which may be more challenging to reverse. For established NVE, cross-sectional OCT may better predict risk of bleeding than clinical assessment and help guide vitreo-retinal surgical management.
Summary
What was known before
-
Structural OCT is useful to assess ILM breach. Neovascular lesions below the ILM are more likely to regress than lesions above the ILM following laser treatment. The size of neovascular lesions may predict the risk of vitreous haemorrhage.
What this study adds
-
Intra retinal lesions with ILM disruption but no breach are at high risk of progression. Lesions on top of a large vein with a small breach may be at risk of progression. Larger intra retinal lesions with no breach may not be at risk of progression.
References
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36.
Early treatment diabetic retinopathy study research group. Grading Diabetic retinopathy from stereoscopic colour fundus photographs- An extension of the Modified Airlie House Classification. ETDRS Rep. Number 10 Ophthalmol. 1991;98:786–99.
Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81:383–96.
Lee CS, Lee Y, Sim DA, Keane PA, Mehta H, Zarranz-Ventura J, et al. Re-evaluation of the definition of intraretinal microvascular abnormalities and neovascularization elsewhere in diabetic retinopathy using optical coherence tomography and fluorescein angiography. Am J Ophthalmol. 2015;159:101–10.
Davis MD. The natural history of diabetic retinopathy. Trans Am Acad Ophthalmol Otolaryngol. 1968;72:237–40.
Dobree JH. Proliferative diabetic retinopathy: evolution of retinal lesions. Br J Ophthalmol. 1964;48:637–49.
Cho H, Alwassia AA, Regiatieri CV, Zhang JY, Baumal C, Waheed N, et al. Retinal neovascularisation secondary to proliferative diabetic retinopathy characterised by spectral domain optical coherence tomography. Retina. 2013;33:542–54.
Kanski JJ, Bowling B. Clinical ophthalmology: a systemic approach. 7th ed 2011, Elsevier. Chapter 13, 543
Imesch PD, Bindley CD, Wallow IHL. Clinicopathologic correlation of intraretinal microvascular abnormalities. Retina. 1997;17:321–32.
Chang SL, Leonard-Martin TC, Feman SS. Relationship between IRMA and diabetic neovascularisation. Invest Ophthalmol Vis Sci. 1995;36:2235–202. S483 abstract
de Carlo TE, Bonini Filho MA, Baumal CR, Reichel E, Rogers A, Witkin AJ, et al. Evaluation of preretinal neovascularization in proliferative diabetic retinopathy using optical coherence tomography angiography. Opth Surg Lasers Imag Retina. 2016;47:115–1.
Pan J, Chen D, Yang X, Zou R, Zhao K, Cheng D, et al. Characteristics of neovascularization in early stages of proliferative diabetic retinopathy by optical coherence tomography angiography. Am J Ophthalmol. 2018;192:146–56.
Muqit MM, Stanga PE. Fourier domain optical coherence tomography evaluation of retinal and optic nerve head neovascularisation in proliferative diabetic retinopathy. Br J Ophthalmol. 2014;98:65–72.
Chatziralli IP, Sergentanis TN, Sivaprasad S. Prediction of regression of retinal neovascularisation after pan-retinal photocoagulation for proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:1715–21.
Russell JF, Shi Y, Scott NL, Gregori G, Rosenfeld PJ. Longitudinal angiographic evidence that intraretinal microvascular abnormalities can evolve into neovascularisation. Ophthalmol Retina. https://doi.org/10.1016/j.oret.2020.06.010 2020.
Ishibazawa A, Nagaoka T, Yokota H, Takahashi A, Omae T, Song YS, et al. Characteristics of retinal neovascularization in proliferative diabetic retinopathy imaged by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:6247–55.
Author information
Authors and Affiliations
Contributions
JD: Data collection and analysis, interpreted results, revised the manuscript, approved final manuscript version, confirms full access to the data and final responsibility to submit for publication. DB: Data collection, interpreted results, revised the manuscript, approved final manuscript version. OC: Data collection, interpreted results, revised the manuscript, approved final manuscript version. JT: Conceived the work, interpreted results, revised the manuscript, approved final manuscript version
Corresponding author
Ethics declarations
Conflict of interest
JD has received a travel grant from Alimera. No competing financial interest in this work is reported. JT serves on advisory boards for Bayer, Novartis, Allergan, Alimera, and participated in research for Bayer, Novartis, Allergan, Alimera, Roche and Boehringer Ingelheim. DB and OC report no competing financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
DaCosta, J., Bhatia, D., Crothers, O. et al. Utilisation of optical coherence tomography and optical coherence tomography angiography to assess retinal neovascularisation in diabetic retinopathy. Eye 36, 827–834 (2022). https://doi.org/10.1038/s41433-021-01544-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01544-8