Abstract
Ocular surface squamous neoplasia (OSSN) is the most common ocular tumour with an incidence ranging from 0.03 to 1.9 per 100,000 persons/year. The diagnosis is made on clinical suspicion and confirmed with anterior-segment optical coherence tomography (AS-OCT), cytology, or histology. The purpose of this review is to provide an overview of the management options available for OSSN and review their success and recurrence rates. Surgery is the gold standard for the management of small OSSN lesions. With the increased use of less invasive diagnostic modalities such as AS-OCT and cytology, there has been a move to use topical therapies for the management of OSSN. The most commonly used agents are interferon-α2b (IFN), mitomycin-C (MMC) and 5-fluorouracil (5FU). They have been shown to have similar resolution and recurrence rates but differ in cost and side effect profile. IFN has the lowest side effect profile, but is also the most expensive, whereas MMC has the greatest surface toxicity and is priced midway between the three. 5FU is the cheapest of the three topical agents with less surface toxicity than MMC. Radiotherapy is mostly employed as adjuvant therapy. Newer novel therapies are available but have not been widely adopted as mainstream therapy due to cost and lack of clinical evidence. OSSN has the benefit of many management options. No single modality has been shown to superior and some patients will need the use of combination therapy to achieve an optimal clinical outcome.
摘要
眼表鳞状细胞癌 (Ocular surface squamous neoplasia, OSSN) 是最常见的眼部肿瘤, 发病率为0.031.9 / 10万人/年。临床表现疑似的病例, 进一步由前节相干光断层扫描 (anterior-segment optical coherence tomography, AS-OCT) 、细胞学或组织学进行确诊。
本综述的目的是针对OSSN可行的治疗策略进行概述, 并阐述它们的成功率和复发率。
手术是治疗小病灶OSSN治疗的金标准。随着AS-OCT和细胞学等侵入性较低的诊断方法的使用越来越多, 局部滴用眼药的治疗OSSN已经成为一种趋势。最常用的药物是干扰素-α2b (interferon-α2b, IFN)、丝裂霉素- c (mitomycin-C, MMC)和5-氟尿嘧啶(5-fluorouracil, 5FU)。它们具有相似的治疗率和复发率, 但在成本和副作用方面有所不同。IFN的副作用最低, 但也是最贵的, 而MMC的表面毒性最大, 价格介于三种药物之间。5FU是三种眼药中最便宜的, 表面毒性较MMC小。放射治疗主要用于辅助治疗。新的治疗手段是可行的, 但由于成本及缺乏临床证据, 并没有被广泛采用作为主流疗法。
OSSN受益于许多治疗以及管理策略。没有一种单一的治疗方式显示出优越性, 一些患者需要使用联合治疗以达到最佳的临床结局。
Similar content being viewed by others
Introduction
Ocular surface squamous neoplasia (OSSN) is the most common ocular tumour with an incidence ranging from 0.03 to 1.9 per 100,000 persons/year [1]. The main associated risk factors are ultraviolet-B (UVB) radiation, human papilloma virus (HPV) and human immunodeficiency virus infection [1]. There are two main patterns of disease presentation, an older white male population with UVB as the primary risk and younger female population where HIV and HPV are more prevalent [1].
Patients typically present with non-specific symptoms such as redness, ocular irritation and visual impairment if the visual axis is involved [1]. Clinically OSSN appears as a pearly grey mass with a variable degree of pigmentation, vascularity and leukoplakia [2]. Confirmation of diagnosis has traditionally been by biopsy, but newer less invasive techniques have been employed in recent years such as anterior-segment optical coherence tomography (AS-OCT) and impression cytology (IC) [2].
Management of OSSN can be divided into surgical and medical management. A standard of care survey conducted in the United States in 2003 showed surgery as the mainstay of therapy [3]. Respondents felt that there was insufficient literature on the use of topical medical therapy and those using topical therapy favoured the use of mitomycin-C (MMC) [3]. A follow-up study was conducted in 2012, which showed that surgery remained the mainstay of therapy, but that that there had been a significant increase in the use of topical therapy [4]. The topical therapy of choice had changed from MMC to interferon-α (IFN) [4].
The purpose of this review is to provide an overview of the management options available for OSSN and review their success and recurrence rates.
Surgical management
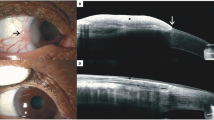
Surgical excision of OSSN remains the gold standard of care for tumours that occupy four clock hours or less of the limbus, or have a basal diameter of 15 mm or less [5]. The ‘no touch technique’, described by Shields et al. [6] in 1997, is the preferred technique for surgical removal [5,6,7]. The basic principles of this technique is to remove the tumour with macroscopically clear margins while minimising the risk of microscopic seeding. There is no universal consensus on the optimal margin used during surgery. Shields et al. [6] suggested a margin of 4–5 mm in their original publication of the technique, however a margin of 3–4 mm is more commonly used [2, 8,9,10,11]. Margin involvement occurs due to the spread of the tumour along the basal layer of the conjunctiva, which is not visible when delineating macroscopic tumour margins [12]. Various ancillary techniques have been employed to aid in the identification of the macroscopic tumour margins. Vital dyes, such as methylene blue and rose-bengal, can be used to stain the tumour and delineate margins (Fig. 1a) [13]. Anterior-segment OCT has been well described for the diagnosis of OSSN [14]. Recently, a combination of pre-operative and intra-operative OCT images have been used to successfully identify the extent of the tumour (Fig. 1b), thereby ensuring adequate margins during surgery [15].
OSSN involves the conjunctiva and commonly extends onto the cornea [2]. A 4-step approach is recommended to ensure removal of the tumour in one piece and to minimise the risk of microscopic tumour seeding (Fig. 2) [6]. The tumour is first approached from the corneal aspect with an alcohol assisted epitheliectomy [6]. An alcohol soaked cotton bud is applied to the epithelium until it becomes grey in appearance [6]. A crescent blade is then used to gently scrape the epithelium, creating a 2 mm clear margin around the tumour, scraping from the centre of the cornea towards the limbus [6]. The tumour will typically lift off easily as this is only an extension of the mass and not invasion of the cornea. It is important not to violate Bowman’s membrane, as this will result in scarring.
The second step is to mobilise the conjunctival aspect of the tumour. The 3–4 mm margins are marked and conjunctiva incised to expose bare sclera. An attempt is made to undermine the conjunctiva under the mass. If there is scleral invasion, a lamellar sclerectomy of 0.2 mm depth is performed starting 2 mm from the tumour margin and ending at the limbus [6]. If the tumour extends more than 5 mm posterior from the limbus, it may be necessary to isolate the underlying extra-ocular muscle to prevent inadvertent surgical trauma [6]. Once the cornea and conjunctival dissection meets at the limbus, the tumour is shaved off in one piece. The specimen should be placed on a cardboard or sponge carrier with orientation guides for the pathologist [6]. This is important for reporting on the margins. The corneal margin is often involved on histology in cases with corneal extension, but as this area is treated with alcohol and the mass removed with 2 mm margins this may not be clinically relevant [2]. With the tumour removed and bare sclera exposed, haemostasis can be achieved before commencing with adjuvant cryotherapy.
Cryotherapy is the third step of surgery causing thermal destruction of cells and vasculature, and is therefore essential for the elimination of residual tumour cells [2]. Cryotherapy is applied to the free conjunctival margin and limbus in two freeze-thaw cycles [2]. It is not necessary to apply cryotherapy to the base of the lesion as this has been excised with macroscopically clear margins. There are various factors affecting the rate of the cryoprobe freeze, and therefore it is advised to aim for a 2 mm freeze around the probe for the conjunctiva and 1 mm for the limbus rather than using a fixed duration of freeze [2]. It is important to lift the conjunctiva off the globe and apply the cryotherapy to the inside edge of the tissue while avoiding the underlying sclera [6]. Excess freezing can cause anterior uveitis, cataract, hypotony, corneal neovascularisation and haemorrhage [2, 5].
The last step of surgery is closure of the scleral defect. The defect can be left to heal by primary intention, however, if the defect is large a conjunctival autograft or amniotic membrane transplant can be used [2, 16]. Primary limbal stem cell transplants have been described in patients that undergo surgery for extensive OSSN, in an attempt to prevent limbal stem cell failure [17, 18]. The main complications of surgery include recurrence, infection, scarring and limbal stem cell failure [2, 5, 19].
Intra-operative microscopic seeding is minimised by employing several intra-operative techniques. Care should be taken not to touch the tumour directly, and any instrument used to manipulate the conjunctiva within the cut margin, should not touch the conjunctiva outside the cut margin [5]. This implies that two sets of conjunctival forceps are required, with the first being removed from the set once the tumour has been removed. To further minimise the risk of microscopic spread, the use of balanced salt solution should be avoided, and the surgical field should remain dry [5].
Recurrence after surgery predominantly occurs within the first 2 years and ranges from 0 to 56% [7, 10, 20, 21]. This rate is primarily affected by the state of the excision margin on histology and use of adjuvant therapy [8, 10, 22, 23]. Residual tumour involvement of the cut margin has consistently been shown to increase the risk of recurrence [2, 7,8,9]. Erie et al. [12] showed a recurrence rate of 5% when margins were clear vs 53% when margins were involved in patients with conjunctival intra-epithelial neoplasia (CIN). Cryotherapy has consistently been shown to decrease the risk of recurrence [10, 22, 23]. Despite this, a case series by Waddell et al. [9] used surgery with 3 mm margins and no cryotherapy as their treatment modality. Their cohort consisted of a young patient population with a high prevalence of HIV, and their recurrence rate was 3% with a median follow-up of 32 months [9]. This may highlight the importance of a meticulous surgical technique and possible effect of a younger demographic on recurrence.
Adjuvant therapy in patients with positive margins include topical therapy for positive radial margins and radiotherapy for deep margin involvement [11, 22]. Adjuvant IFN has been shown to reduce recurrence rates in patients with positive surgical margins to the level of patients with non-involved margins [22]. Adjuvant proton radiotherapy was shown to reduce the rate of recurrence in patients with squamous cell carcinoma (SCC) [21].
When tumours are too large or invasive, more extensive surgical options need to be employed. Enucleation is done when the tumour invades the globe (more commonly seen with the mucoepidermoid and spindle cell variants of OSSN) and the tumour margins are visible [5, 24]. During the surgery the peritomy is extended to surround the tumour to create a 3–4 mm clear margin [5]. The remaining conjunctival margin undergoes double cryotherapy before the prosthesis is inserted [5]. It may be necessary to use amniotic membrane or mucous membrane grafts for adequate closure [5]. Tumours that invade the orbit require exenteration [5]. If the anterior lamellae of the lid is spared, then a lid sparing exenteration can be performed, however, if the posterior lamellae is involved then an eyelid-removing exenteration is performed [5].
Surgery remains the gold standard for smaller tumours. It is not the most cost effective treatment option, but yields immediate resolution and has recurrence rates similar to medical management.
Medical management
With the advent of less invasive diagnostic modalities such as AS-OCT, confocal microscopy and impression cytology, there has been a move to less invasive management options. The three leading topical treatments used today are interferon-α2b (IFN), 5-fluorouracil (5FU) and mitomycin-C (MMC) (Table 1) [25]. Topical therapy avoids the risks associated with surgery and has the benefit of treating the entire ocular surface [25]. For larger tumours that extend onto the cornea, topical therapy avoids the risk of damage to cornea during surgery which may have significant visual consequences [26]. The main disadvantage of these therapies is the longer duration of therapy and need for compliance [27].
Mitomycin-C
MMC is a non-cell-cycle dependant alkylating agent derived from Streptomyces caespitosus [25, 28]. It has a number of applications in ophthalmology that include intra-operative use during glaucoma surgery, refractive surgery and the treatment of OSSN [28]. MMC preferentially effects rapidly dividing cells and induces DNA damage through two main pathways, aerobic and anaerobic [28, 29]. In aerobic conditions, mostly found in ophthalmological applications, it generates free radicals that directly damage DNA and proteins [28]. In the anaerobic environment it causes alkylation of DNA and inhibits RNA and protein synthesis [28]. DNA is therefore either damaged by free radicals or through alkylation resulting in apoptosis [28, 30]. These MMC related changes to the ocular surface may persist for more than 8 months, which is important to bear in mind when interpreting impression cytology specimens after MMC use [30].
MMC can be used as neoadjuvant, adjuvant or primary therapy. It can used in two concentrations of 0.02 or 0.04%, both dosed four times a day [29, 31,32,33,34,35]. Due to toxicity, MMC is generally given in cycles, where active drug is administered followed by a drug holiday. The most commonly used cycle is 1-week of MMC followed by a 1-week drug holiday [29]. Less commonly used dosing schedules include 1 week of MMC drops followed by a 3-week drug holiday, or 14 days of continuous MMC (usually given at a lower dose of 0.02%) [34, 35].
Neoadjuvant MMC has been used to decrease tumour size before commencing surgery, thereby minimising the risks associated with surgery such as limbal stem cell failure [36,37,38]. Adjuvant MMC can be used intra or post-operatively. Siganos et al. [39] described a series of patients who underwent surgical excision for OSSN with intra-operative MMC 0.02% applied to the bare sclera for 2.5 min [39]. After a thorough rinse with balanced salt solution, the conjunctiva was closed to leave no more than 2–3 mm of bare sclera adjacent to the limbus [39]. In three of the seven OSSN cases, margins were involved, and recurrence developed in only one of these patients with a mean follow-up of 15 months [39]. Several studies have used adjuvant MMC in the post-operative period to prevent recurrence [31,32,33, 40]. Various regimens were used and even in the absence of adjuvant cryotherapy and surgical margin involvement in up to 50% of cases, there were no recurrences reported with follow-up of up to 5 years [31,32,33, 40]. Zaki et al. [40] added ciclosporin to their post-operative regimen of MMC to reduce the severity of side effects. Altogether this highlights the benefit of adjuvant MMC, where recurrence is prevented even in cases with positive surgical margins where adjuvant cryotherapy was not used. This may play an important role in resource constrained settings where cryotherapy is not available.
When used as primary therapy, MMC has been found to cause tumour resolution in 76–100% of cases, with a recurrence rate of 0–35% [26, 34,35,36,37, 41,42,43,44,45,46]. Frucht-Pery et al. [26] were the first to describe the use of MMC for patients with OSSN recurrence after surgery (n = 16). They had a 76% response rate and recurrence rate of 35% [26]. They did not have a standard treatment regimen and did not have the benefit of newer diagnostic modalities such as AS-OCT to ensure complete response before stopping therapy [26]. Danniel et al. [29] used MMC in cycles of 1 week on, 1 week off. They had a resolution rate of 90% (n = 18) with a mean of two cycles and a recurrence rate of 20% with a mean follow-up of 13 months [29]. They used a strength of either 0.02% or 0.04% and did not find a difference in the response or recurrence rate between the two concentrations [29]. A lower concentration of 0.02% was described by Ballalai et al. [34] as primary therapy for newly diagnosed or recurrent OSSN (n = 23). The defined 14 days of therapy as one cycle and had complete resolution in all cases after two cycles [34]. Mean follow up was 46 months, and one patient developed a recurrence at month 24 [34]. Besley et al. [35] used MMC 0.04% in cycles of 1 week of drops followed by a 3-week drug holiday for non-invasive OSSN. They had a 79% (n = 102) response rate with a mean of 3.3 cycles and 15% recurrence at a mean of 23 months. MMC has also been shown to be effective as primary therapy in advanced tumours. Shields et al. [44] reviewed the effect of MMC 0.04% for extensive OSSN with a basal dimeter of >8 mm (n = 10). They used a mean of 3 cycles (1 week on, 1 week off), and had complete resolution in eight out of ten cases with no recurrence during a mean follow-up period of 22 months [44]. These studies highlight the efficacy of MMC as primary therapy with no specific regimen yielding superior outcomes.
MMC causes the greatest surface toxicity of the topical therapies, as it causes non-cell cycle dependent damage [2, 25]. The most common side effects include limbal stem cell deficiency, punctal stenosis, ocular irritation, lid toxicity, conjunctival injection, epiphora, recurrent corneal erosion and punctate keratopathy [26, 28, 29, 31, 34,35,36, 38, 42, 46]. Side effects have been severe enough to result in cessation of therapy in 5–18% of patients [26, 29, 35, 45]. In one study, 17% of patients using MMC 0.04% developed punctal stenosis that required surgical intervention [32]. To prevent punctal stenosis, many studies advised occlusion of the inferior canaliculi after drop administration or use of punctal plugs in the lower canaliculus [31, 34, 43,44,45]. Corneal epithelial erosions have occurred in up to 17% of patients and can develop up to 24 months after treatment [34]. Overall, side effects are seen less commonly with MMC than with 5FU (41 vs 69%, p < 0.003), but the severity of side effects that require intervention is higher with MMC [32].
The benefit of MMC is that the duration of therapy is shorter than the other topical agents and it is more cost effective than IFN [35]. The disadvantages are that the drops are not stable and therefore need to be compounded weekly and stored refrigerated. MMC is also associated with the most severe surface toxicity of the topical agents [34, 35].
5-fluorouracil
5FU is a pyrimidine analogue that has primarily been used in glaucoma filtering surgery and for the management of OSSN [47]. It has cell cycle dependent toxicity through several mechanisms including, incorporation of a thymidine analogue into DNA, disruption of RNA and ribosomal RNA synthesis, and indirect disruption of the actin cytoskeleton [47].
The use of 5FU for the management of OSSN was first described by De Keizer in 1986 [48]. It can be used as neoadjuvant, adjuvant or primary therapy. As with MMC, 5FU is usually given in cycles of active drug followed by a drug holiday. As 5FU is cell cycle dependent and primarily affects rapidly dividing cells, it has less surface toxicity than MMC [25]. It is usually given at a dose of 1% with cycles consisting of 1 week on and 3 weeks off, 1 month on and 1 month off or 3 days on and 4 days off.
Neoadjuvant 5FU has been described in one case series where it was used as primary therapy for multifocal OSSN [49]. It caused resolution in 44% of cases, and for the remain cases decreased the tumour burden to allow for successful surgery without recurrence [49]. 5FU is the least reported topical agent for use as neoadjuvant therapy for OSSN [49,50,51].
Yeatts et al. [52] used 5FU 1% as adjuvant therapy after surgical excision (n = 6). They continued application until an epithelial defect developed (14–21 days) and waited for epithelial healing before commencing the next cycle [52]. They had a response in all patients with recurrence in 33% [52]. A larger case series (n = 89) used adjuvant 5FU 1% administered four times a day for 2 weeks after epithelial healing [32]. They had only a single recurrence in a patient who did not complete his 2-week of 5FU, with an overall median follow-up of 34 months [32]. Gichuhi et al. [53] conducted a randomised placebo controlled double blind study to compare the efficacy of adjuvant 5FU 1%, administered four times a day for 1 month after surgical excision without adjuvant cryotherapy. They found that adjuvant 5FU significantly reduced 1-year recurrence rates (11 vs 36%, p = 0.01) [53]. They did not find that surgical margin involvement was a significant factor to recurrence [53]. This is a significant finding and could be employed in settings that do not have access to cryotherapy to reduce recurrence rates.
When used as primary therapy (Fig. 3), 5FU has been found to cause tumour resolution in 82–100% of cases, with a recurrence rate of 7–43% [49,50,51, 54,55,56]. Al-Barrag et al. [56] used a 5 mg subconjunctival injection of 5FU at the end of surgery (diagnostic incision biopsy) followed by a regimen of 5FU1% administered four times a day for 4 days, with a 30 day drug holiday. They had resolution in all cases (n = 15) with a mean of 6 cycles and had recurrence in one patient that resolved with additional cycles [56]. A larger case series described the use of 5FU 1% cycled 1 week on and 3 weeks off (median of 4 cycles) with an 82% (n = 36) response rate and 11% recurrence rate [50]. Two of the recurrences were at 3 months and therefore were more likely incomplete treatment rather than true recurrences. Parrozzani et al. [49] conducted a study where 5FU 1%, cycled 1 month on 1 month off, was used as primary therapy for OSSN in 41 patients. They had complete tumour regression in 83% of cases with a mean of 1.5 cycles [49]. They also found that multi-focality (p = 0.023) and tumour thickness >1.5 mm (p = 0.045) were associated with a poorer response to therapy [49]. They had a mean follow-up period of 105 months and a 12% recurrence rate [49]. Venkateswaran et al. [51] compared the efficacy of IFN and 5FU. They used a dosing schedule of 5FU 1% four times a day for a week with a 3-week drug holiday, and IFN 1 million IU/ml four times a day until resolution [51]. They had a 96% response rate with 5FU with a median of 4 cycles, and 81% response rate with IFN with a median treatment duration of 4 months [51]. With multivariate analysis this difference was not found to be significant [51]. Recurrence rates were 12% with 5FU and 5% with IFN (p = 0.46) with a mean follow-up of 16 months [51]. There were more side effects noted in the 5FU cohort with one patient stopping therapy due to eyelid pain [51]. It is important to note that no additional medication was used to prevent surface toxicity in either group. Lastly, HIV infection has been found to have no effect on the response to 5FU therapy [57].
5FU has less surface toxicity than MMC, as it has cell cycle dependent toxicity [47, 49]. Side effects are mostly mild and transient, including punctate epithelial erosions and ulceration, lid toxicity and keratoconjunctivitis [47, 49, 52, 55, 56, 58]. Lid toxicity was minimised in some studies by applying an ointment to the lid margins before drop instillation [49]. One study showed adverse effects in 57% of patients with 6% not completing a cycle due to side effects [58]. This study did not use any additional medication to minimise drug toxicity.
The advantages of 5FU are that is relatively inexpensive, stable at room temperature and has less side effects than MMC [55]. The disadvantages include longer duration of therapy (compared to surgery and MMC) and greater toxicity than IFN [58].
Interferon-α
Interferons are cytokines with anti-viral, antiproliferative, immunomodulatory and cytotoxic properties [59]. There are three different types of IFN of which type 1 (IFN-α, IFN-β, IFN-γ) has activity against infections and neoplasms [59]. IFN-α has been used for solid tumours since 1971 and was first described for use in OSSN by Maskin et al. [60] in 1994 [61]. Since then recombinant IFN-α2b (IFN) has been widely used for ocular surface disease including OSSN and limbal stem cell failure [59]. Although the exact mechanism of action of IFN in OSSN is not fully understood, a number of properties have been described [59].
The anti-tumour properties of IFN are attributed to anti-viral activity, direct anti-tumour effects, immunomodulatory and anti-angiogenic effects [59, 61]. IFN has shown to be effective against viral induced malignancies such as hepatitis induced hepatocellular carcinoma and herpes simplex virus associated Kaposi’s sarcoma [61]. OSSN has been associated with HPV infection and therefore the anti-viral activity of IFN has been thought to play a role in the anti-tumour effects in OSSN [1, 61]. This effect has however not been found to only be dependent on the presence of HPV [62]. The direct anti-tumour effect is achieved by prolonging the cell cycle of tumour cells resulting in a cytostatic effect [61]. It inhibits cellular enzymes, resulting in a deficiency of metabolites which inhibits tumour growth [61]. Lastly, IFN has been shown to have pro-apoptotic effects on squamous cell cancers [61]. The indirect effect is achieved by enhancing the bodies immunomodulatory response to cancer cells [61]. IFN up-regulates the major histocompatibility complex on malignant cells, increases the production of antibodies and enhances the cytotoxicity of immune cells, thereby augmenting the immune systems elimination of tumour cells [61]. For this reason, It has been suggested that IFN rather be used in immune-competent patients as part of the anti-tumour effect is from the enhanced immune modulation [19]. The evidence for this is based on only two case reports [63, 64]. Further studies are needed to determine the efficacy of IFN in immune-compromised patients. Altogether, IFN is effective at removing associated HPV infection, slowing tumour growth and augmenting apoptosis, and increasing the effectiveness of the immune system against cancer cells.
IFN can be used topically or intralesional as neoadjuvant (immuno-reduction), adjuvant (immune-prevention) or as primary therapy (immunotherapy) [27, 59, 65, 66]. The most commonly used topical regimen is 1 million IU/ml, administered four times a day for 1–3 months after clinical resolution of the tumour [27, 65, 67, 68]. Extending treatment after clinical resolution ensures elimination of any residual tumour cells. The average duration of topical therapy is 5 months, but can continue for more than a year [25, 27, 65, 69]. Doses as high as 2–3 million IU/ml have also been used with similar outcomes [27, 67, 70]. Galor et al. [67] compared the outcomes of 1 and 3 million IU/ml topical therapy and found that there was no significant difference in tumour response and that the additional cost was not justified. IFN can be combined with topical retinoic acid 0.01% [71]. It is thought that retinoic acid may have a synergistic effect with IFN [71].
Intralesional or subconjunctival injection of IFN are mostly given at a dose of 3 million IU in 0.5 ml, administered weekly until tumour resolution [27, 68]. This is injected around the base of the lesion in a single or divided injection and most commonly requires 4–5 injections [27]. The initial injection frequency used was based on systemic treatment algorithms and given three times a week [68]. This was however not found to be superior to a more convenient weekly dosing schedule [68]. Doses as high as 10 million IU have been described and are administered monthly [27, 65]. Injections have benefits over topical therapy in that there is improved patient compliance, a shorter course of treatment and there is no need to compound the IFN [25, 68]. Injections do however have a greater side effect profile that includes flu like symptoms in 33% of patients [65, 68, 69]. This can generally be prevented by the concomitant use of oral paracetamol, 1 g taken 4 hourly as needed [27, 68]. Karp et al. [68] investigated the benefit of combining topical and subconjunctival therapy and found no additional benefit with a similar time to resolution compared to individual applications.
IFN can be used in the neoadjuvant setting to reduce tumour size before surgery [65, 66]. In a case series by Kim et al. [65] 12 of 18 eyes with giant OSSN had complete tumour resolution with IFN, the remaining six patients had sufficient immune-reduction to allow for successful surgery. No cases had recurrence during a mean follow-up period 11 months [65]. IFN can also be used as adjuvant therapy after surgery if the surgical margins are involved on histology [27]. A dose of 1 million IU/ml administered four times a day is typically used for 2 months [27].
When used as immunotherapy studies have shown tumour resolution in 81–100% of cases, with a recurrence rate of 0–9% [8, 35, 67,68,69,70,71,72,73,74,75]. Kaliki et al. [72] showed that IFN was effective against pigmented OSSN with a 100% resolution (average of 2 months treatment) and no recurrence over a mean follow-up of 13 months [72]. This is important in the South African context as the prevalence of pigmented OSSN is 2% in white patients and up to 53% in black patients [72]. Surgery has been compared to topical IFN therapy, and showed similar recurrence rates of 5 and 3% for surgery and IFN respectively (p = 0.08) [8, 76]. Kusumesh et al. [36] compared the efficacy of topical IFN and MMC. They found a similar response rate of 89 and 92% for IFN and MMC respectively, with only one recurrence in the IFN group [36]. They found that MMC resulted in faster tumour resolution (1.5 months for MMC vs 3.5 months for IFN, p < 0.005) [36]. There was a much higher rate of side effects with MMC (88%) compared to IFN (12%), although none were severe or resulted in cessation of therapy [36]. IFN has also been successfully used in a series with socket involving OSSN, where all cases avoided the standard of care, exenteration [77].
Topical IFN is well tolerated with minimal side effects such as conjunctival injection, follicular conjunctivitis, corneal erosion, corneal ulcer and epithelial microcysts [27, 35, 65, 67,68,69,70,71, 74]. These side effects are usually mild and do not result in cessation of treatment [8, 35, 69]. Although it is the best tolerated of the topical therapies (IFN, 5FU, MMC), it is much more expensive and requires refrigeration for storage [27, 36, 51, 69]. Once compounded, IFN remains stable for 30 days [25].
Radiotherapy
Radiotherapy can be divided into external beam radiotherapy (EBRT) and brachytherapy (BT) [78]. Proton and electron EBRT are favoured in OSSN as they limit radiation exposure to the tumour and minimise negative effects on the normal surrounding tissue (cornea, lens and retina) [79]. Several case reports have demonstrated complete tumour resolution with minimal side effects in patients with extensive tumours, including ocular invasion, that would otherwise have undergone enucleation [79,80,81]. In cases with severe orbital invasion, it has been used for palliation [82].
Brachytherapy is used as adjuvant therapy after surgical excision and is applied directly to the ocular surface after epithelial healing is complete [78]. Three main isotopes are used, including strontium-90 (Sr-90), iodine-125 (I-125) and ruthenium-106 (R-106) [78]. A large case series in South Africa used Sr-90 as sole adjuvant therapy after surgical excision and had a 12% recurrence rate with a median follow-up of 27 months [83]. They reported dry eye as the only adverse event [83]. Walsh-Conway et al. [84] reported favourable outcomes with I-125, with no recurrences over a mean follow-up of 23 months. The adverse events reported in this series included dry eyes, corneal ulceration and peripheral corneal vascularisation [84]. Lastly, R-106 has been shown to be effective in a French case series, with no recurrences over a mean follow-up of 48 months [85].
Novel therapies
Photodynamic therapy
Photodynamic therapy uses a combination of a photosensitising agent (verteporfin) and diode laser to produce oxygen free radicals that result in vascular occlusion that promotes tumour destruction [86]. In a pilot study it has caused tumour resolution in 100% of cases that are smaller than the laser spot size of 5 mm, with no recurrence at a year [86]. In one patient with a tumour larger than the spot size, multiple treatments were given, but they did not achieve complete tumour resolution [86]. There were minor reversible side effects that included chemosis, conjunctival haemorrhages and foreign body sensation [86]. Cekic et al. [87] reported a case of giant OSSN covering 10 clock hours of the limbus and 50% of the cornea. By using overlapping spots in the treatment, they had complete tumour resolution after two treatments with no recurrence with 13 months of follow-up [87]. There was exposure of bare sclera with tumour resolution that required a conjunctival autograft [87]. A limitation to adoption of this modality is the high cost of the photosensitising agent and limited evidence in the literrature [86].
Anti-VEGF
Anti-vascular endothelial growth factor (VEGF) has been used successfully in the treatment of several malignancies such as colon, rectal and breast cancer [88]. It is also a frequently used agent in ophthalmology for retinal oedema and neovascularisation [89]. Subconjunctival anti-VEGF injections for OSSN have been described in several small case series, with resolution shown in up to 60% of cases [88,89,90]. These series were small and it appears to cause a reduction in the conjunctival component of tumours, giving it a possible role as neoadjuvant therapy for tumour reduction before surgery. One case series describes the use of topical anti-VEGF therapy, which showed tumour reduction of both the conjunctiva and corneal components of the tumour [91]. No significant side effects were reported in any of these series [88,89,90,91].
Cidofovir
Cidofovir is an anti-viral drug with activity against double stranded DNA viruses [92]. A single case series from Australia reported resolution of treatment resistant OSSN in 83% of cases treated with topical Cidofovir 0.25% administered three times a day for 4–9 weeks [92]. Part of the rationale of using this drug was that it is effective against HPV, however, in the series there was only one patient that tested positive for HPV-16 [92]. Future use of this drug will need to be examined further in larger studies with longer follow-up.
EGFR Inhibitors
Epidermal growth factor receptor (EGFR) has been shown to be overexpressed in squamous cell carcinoma of the head and neck [93,94,95]. The level of EGFR expression has also been correlated with tumour size, stage and recurrence [93]. It has therefore been identified as a possible target for therapy in OSSN. A case series showed a significant response to systemic EGFR inhibitors in two elderly patients with advanced orbital extension of OSSN [95]. A more recent case report of SCC of the lacrimal sac showed complete resolution of the tumour with 3 cycles of an EGFR inhibitor combined with systemic chemotherapy [96].
The high cost of these agents has limited their accessibility and clinical use. Their potential application in OSSN therapy could be directed to neoadjuvant therapy for large conjunctival or orbital tumours to reduce surgical morbidity. Future therapies may involve the development of topical EGFR inhibitors that could be combined with existing topical therapies such as 5FU and IFN.
Checkpoint inhibitors
The adaptive immune system has the potential to remove cancer cells through the combined effect of CD4 and CD8 T-cells, and B-cells [97]. In order to maintain checks and balances in the system, and prevent auto-immune responses, co-inhibitory receptors are present and act as negative regulators in an immune response [97]. Two of these regulators are Cytotoxic T lymphocyte antigen 4 (CTLA4) and Programmed cell death protein 1 (PD1) [98]. In conditions with persistent antigen stimulation such as cancer and chronic infection, these molecules are upregulated to prevent T-cell exhaustion and modulate the immune response [97]. This had led to the development of checkpoint inhibitors, which block the inhibitory function of CTLA4 and PD1, allowing the immune system to recognise and eliminate cancer cells [96].
Esmaeli et al. [96] describe a positive response with checkpoint inhibitors in patients with advanced SCC in the periocular region. There may also be benefit in combining checkpoint inhibitors, as CTLA4 inhibitors upregulate the expression of PD1, thereby increasing the efficacy of PD1 inhibitors [98]. The disadvantages of these novel systemic therapies are the cost and side effect profiles [96].
Discussion
OSSN is the most common ocular malignancy. It is traditionally managed by surgical excision which also provides tissue for diagnosis. Surgical excision is performed using the well accepted ‘no touch’ technique popularised by Shields et al. [6]. This technique aims to remove the macroscopic tumour, while adequate surgical margins increase the likelihood of removing of any microscopic tumour extension. Even with this technique, margins may still be involved with recurrence rates as high as 29% [10]. For this reason adjuvant therapies have been used to reduce recurrence. The most used adjuvant during surgery is cryotherapy, whereas topical MMC, IFN and 5FU can be used as adjuvant therapy in the post-operative period once the epithelium has healed. Some centres have used this routinely for OSSN patients after surgery, where other centres have only used this when surgical margins are positive on histology [27, 54].
With the advent of less invasive diagnostic modalities such as AS-OCT and impression cytology, there has been a move to the use of topical medical agents in the treatment of OSSN. This is also the treatment of choice when tumour sizes exceed 4 clock hours of the limbus, with the risk of limbal stem cell failure increasing when excising large lesions with 4 mm margins. The benefit of these therapies is that surgery and its potential complications are avoided, the entire ocular surface is treated and convenience. The main disadvantages are the longer duration of management. There are three options for topical medical therapy, MMC, 5FU and IFN (Table 1). All three have similar outcomes in terms of tumour resolution rates and recurrence. The main differences come with cost, storage, and side effect profile. MMC and 5FU are cost effective, whereas IFN is a more expensive option considering the average duration of treatment is 6 months. 5FU has the benefit of room temperature storage whereas MMC and IFN require refrigeration. With a dosing schedule of four times a day, it may be difficult to keep drops refrigerated in resource constrained environments. Lastly, IFN has the lowest toxicity profile of the three followed by 5FU and MMC. Overall, IFN is the better option when cost is not an issue, but 5FU remains the best option when considering all three parameters. Long-term follow up is extremely important as the mean time to tumour recurrence is mostly within 2 years of resolution [7, 10, 20, 21, 35, 51, 70].
Radiotherapy is largely reserved as adjuvant therapy for deep tumour margin involvement (BT) and for palliation (EBRT) in extensive tumours.
Novel therapies that show the greatest promise are the EGFR and checkpoint inhibitors. They provide a neoadjuvant avenue of care for patients that would otherwise need more extensive surgery. Firstly, they could be used in patients with large conjunctival masses that extend into the anterior orbit but retain good vision. Reducing the size of these masses could allow for globe saving surgical excision. Secondly patients with invasion beyond the orbit are currently treated with EBRT aimed at palliation. These agents have been found to reduce the size of such extensive lesions, which could make them curable by exenteration [96]. There are limited reports in the literature using these agents and the high cost has limited their adoption.
OSSN has the benefit of many diagnostic and therapeutic modalities. There is no one size fits all in the management of OSSN. Some patients may need a change or combination of therapy to achieve resolution. Smaller tumours can be managed surgically or medically with equal success. Large tumours are preferentially managed by medical therapy to reduce surgical morbidity. The most favourable medical therapy in a developing country is 5FU.
Summary
What was known before
-
Surgical excision using the no-touch technique is the gold standard in the management of ocular surface neoplasia involving four clock hours or less of the limbus.
-
Topical chemo and immunotherapy utilisation has increased with an important role for managing ocular surface neoplasia involving more than four clock hours of the limbus.
What this study adds
-
A comprehensive overview of all treatment modalities for ocular surface neoplasias.
-
A review of newer emergent novel therapies.
References
Hollhumer R, Williams S, Michelow P Ocular surface squamous neoplasia: Population demographics, pathogenesis and risk factors. Afr. Vis. Eye Health. 2020; 79. Available at: http://www.avehjournal.org/index.php/AVEH/article/view/553 [Accessed June 23, 2020].
Basti S, Macsai MS. Ocular surface squamous neoplasia: a review. Cornea. 2003;22:687–704.
Stone DU, Butt AL, Chodosh J. Ocular surface squamous neoplasia: a standard of care survey. Cornea. 2005;24:297–300.
Adler E, Turner JR, Stone DU. Ocular surface squamous neoplasia: a survey of changes in the standard of care from 2003 to 2012. Cornea. 2013;32:1558–61.
Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Surv Ophthalmol. 2004;49:3–24.
Shields JA. Surgical management of conjunctival tumors: the 1994 Lynn B. McMahan lecture. Arch Ophthalmol. 1997;115:808.
Tabin G, Levin S, Snibson G, Loughnan M, Taylor H. Late recurrences and the necessity for long-term follow-up in corneal and conjunctival intraepithelial neoplasia. Ophthalmology. 1997;104:485–92.
Sturges A, Butt AL, Lai JE, Chodosh J. Topical interferon or surgical excision for the management of primary ocular surface squamous neoplasia. Ophthalmology. 2008;115:1297–.e1.
Waddell KM, Downing RG, Lucas SB, Newton R. Corneo-conjunctival carcinoma in Uganda. Eye. 2006;20:893–9.
Sudesh S, Rapuano CJ, Cohen EJ, Eagle RC, Laibson PR. Surgical management of ocular surface squamous neoplasms: the experience from a cornea center. Cornea. 2000;19:278–83.
Kamal S, Kaliki S, Mishra DK, Batra J, Naik MN. Ocular surface squamous neoplasia in 200 patients. Ophthalmology. 2015;122:1688–94.
Erie JC, Campbell RJ, Liesegang TJ. Conjunctival and corneal intraepithelial and invasive neoplasia. Ophthalmology. 1986;93:176–83.
Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39:429–50.
Atallah M, Joag M, Galor A, Amescua G, Nanji A, Wang J, et al. Role of high resolution optical coherence tomography in diagnosing ocular surface squamous neoplasia with coexisting ocular surface diseases. Ocul Surf. 2017;15:688–95.
Karp CL, Mercado C, Venkateswaran N, Ruggeri M, Galor A, Garcia A, et al. Use of high-resolution optical coherence tomography in the surgical management of ocular surface squamous neoplasia: a pilot study. Am J Ophthalmol. 2019;206:17–31.
Palamar M, Kaya E, Egrilmez S, Akalin T, Yagci A. Amniotic membrane transplantation in surgical management of ocular surface squamous neoplasias: long-term results. Eye Lond Engl. 2014;28:1131–5.
Mittal V, Narang P, Menon V, Mittal R, Honavar S. Primary simple limbal epithelial transplantation along with excisional biopsy in the management of extensive ocular surface squamous neoplasia. Cornea. 2016;35:1650–2.
Narang P, Mittal V, Menon V, Bhaduri A, Chaudhuri B, Honavar S. Primary limbal stem cell transplantation in the surgical management of extensive ocular surface squamous neoplasia involving the limbus. Indian J Ophthalmol. 2018;66:1569.
Cicinelli MV, Marchese A, Bandello F, Modorati G. Clinical management of ocular surface squamous neoplasia: a review of the current evidence. Ophthalmol Ther. 2018;7:247–62.
Li AS, Shih CY, Rosen L, Steiner A, Milman T, Udell IJ. Recurrence of ocular surface squamous neoplasia treated with excisional biopsy and cryotherapy. Am J Ophthalmol. 2015;160:213–.e1.
Santoni A, Thariat J, Maschi C, Herault J, Baillif S, Lassalle S, et al. Management of invasive squamous cell carcinomas of the conjunctiva. Am J Ophthalmol. 2019;200:1–9.
Galor A, Karp CL, Oellers P, Kao AA, Abdelaziz A, Feuer W, et al. Predictors of ocular surface squamous neoplasia recurrence after excisional surgery. Ophthalmology. 2012;119:1974–81.
Fraunfelder FT, Wingfield D. Management of intraepithelial conjunctival tumors and squamous cell carcinomas. Am J Ophthalmol. 1983;95:359–63.
Kaliki S, Jajapuram SD, Maniar A, Taneja S, Mishra DK. Ocular surface squamous neoplasia with intraocular tumour extension: a study of 23 patients. Eye. 2020;34:319–26.
Nanji AA, Sayyad FE, Karp CL. Topical chemotherapy for ocular surface squamous neoplasia. Curr Opin Ophthalmol. 2013;24:336–42.
Frucht-Pery J, Sugar J, Baum J, Sutphin JE, Pe’er J, Savir H, et al. Mitomycin C treatment for conjunctival—corneal intraepithelial neoplasia. Ophthalmology. 1997;104:2085–93.
Al Bayyat G, Arreaza-Kaufman D, Venkateswaran N, Galor A, Karp CL. Update on pharmacotherapy for ocular surface squamous neoplasia. Eye Vis. 2019;6:24.
Abraham LM, Selva D, Casson R, Leibovitch I. Mitomycin: clinical applications in ophthalmic practice. Drugs. 2006;66:321–40.
Daniell M, Maini R, Tole D. Use of mitomycin C in the treatment of corneal conjunctival intraepithelial neoplasia. Clin Exp Ophthalmol. 2002;30:94–98.
McKelvie PA. Impression cytology following mitomycin C therapy for ocular surface squamous neoplasia. Br J Ophthalmol. 2001;85:1115–9.
Akpek EK, Ertoy D, Kalayci D, Hasiripi H. Postoperative topical mitomycin C in conjunctival squamous cell neoplasia. Cornea. 1999;18:59–62.
Bahrami B, Greenwell T, Muecke JS. Long-term outcomes after adjunctive topical 5-flurouracil or mitomycin C for the treatment of surgically excised, localized ocular surface squamous neoplasia: Treatment of ocular surface neoplasia. Clin Exp Ophthalmol. 2014;42:317–22.
Chen C. Mitomycin C as an adjunct in the treatment of localised ocular surface squamous neoplasia. Br J Ophthalmol. 2004;88:17–18.
Ballalai PL, Erwenne CM, Martins MC, Lowen MS, Barros JN. Long-term results of topical mitomycin C 0.02% for primary and recurrent conjunctival-corneal intraepithelial neoplasia. Ophthal Plast Reconstr Surg. 2009;25:296–9.
Besley J, Pappalardo J, Lee GA, Hirst LW, Vincent SJ. Risk factors for ocular surface squamous neoplasia recurrence after treatment with topical mitomycin C and interferon alpha-2b. Am J Ophthalmol. 2014;157:287–.e2.
Kusumesh R, Ambastha A, Kumar S, Sinha BP, Imam N. Retrospective comparative study of topical interferon α2b versus mitomycin C for primary ocular surface squamous neoplasia. Comp Study. 2017;36:5.
Rozenman Y, Frucht-Pery J. Treatment of conjunctival intraepithelial neoplasia with topical drops of mitomycin C. Cornea. 2000;19:1–6.
Russell HC, Chadha V, Lockington D, Kemp EG. Topical mitomycin C chemotherapy in the management of ocular surface neoplasia: a 10-year review of treatment outcomes and complications. Br J Ophthalmol. 2010;94:1316–21.
Siganos CS, Kozobolis VP, Christodoulakis EV. The intraoperative use of mitomycin-C in excision of ocular surface neoplasia with or without limbal autograft transplantation. Cornea. 2002;21:12–16.
Zaki AA, Farid SF. Management of intraepithelial and invasive neoplasia of the cornea and conjunctiva: a long-term follow up. Cornea. 2009;28:986–8.
Dogru M, Erturk H, Shimazaki J, Tsubota K, Gul M. Tear function and ocular surface changes with topical mitomycin (MMC) treatment for primary corneal intraepithelial neoplasia. Cornea. 2003;22:627–39.
Gupta A, Muecke J. Treatment of ocular surface squamous neoplasia with Mitomycin C. Br J Ophthalmol. 2010;94:555–8.
Hirst LW. Randomized controlled trial of topical mitomycin C for ocular surface squamous neoplasia. Ophthalmology. 2007;114:976–82.
Shields CL, Naseripour M, Shields JA. Topical mitomycin C for extensive, recurrent conjunctival-corneal squamous cell carcinoma. Am J Ophthalmol. 2002;133:6.
Wilson MW, Hungerford JL, George SM, Madreperla SA. Topical mitomycin C for the treatment of conjunctival and corneal epithelial dysplasia and neoplasia. Am J Ophthalmol. 1997;124:303–11.
Rudkin AK, Dempster L, Muecke JS. Management of diffuse ocular surface squamous neoplasia: efficacy and complications of topical chemotherapy: Management of diffuse ocular surface squamous neoplasia. Clin Exp Ophthalmol. 2015;43:20–25.
Abraham LM, Selva D, Casson R, Leibovitch I. The clinical applications of fluorouracil in ophthalmic practice. Drugs. 2007;67:237–55.
De Keizer RJW, De Wolff-Rouendaal D, Van Delft JL. Topical application of 5-Fluorouracil in premalignant lesions of cornea, conjunctiva and eyelid. Doc Ophthalmol. 1986;64:31–42.
Parrozzani R, Frizziero L, Trainiti S, Testi I, Miglionico G, Pilotto E, et al. Topical 1% 5-fluoruracil as a sole treatment of corneoconjunctival ocular surface squamous neoplasia: long-term study. Br J Ophthalmol. 2017;101:1094–9.
Joag MG, Sise A, Murillo JC, Sayed-Ahmed IO, Wong JR, Mercado C, et al. Topical 5-fluorouracil 1% as primary treatment for ocular surface squamous neoplasia. Ophthalmology. 2016;123:1442–8.
Venkateswaran N, Mercado C, Galor A, Karp CL. Comparison of topical 5-fluorouracil and interferon Alfa-2b as primary treatment modalities for ocular surface squamous neoplasia. Am J Ophthalmol. 2019;199:216–22.
Yeatts RP, Ford JG, Stanton CA, Reed JW. Topical 5-fluorouracil in treating epithelial neoplasia of the conjunctiva and cornea. Ophthalmology. 1995;102:1338–44.
Gichuhi S, Macharia E, Kabiru J, Zindamoyen AM, Rono H, Ollando E, et al. Topical fluorouracil after surgery for ocular surface squamous neoplasia in Kenya: a randomised, double-blind, placebo-controlled trial. Lancet Glob Health. 2016;4:e378–e385.
Yeatts RP, Engelbrecht NE, Curry CD, Ford JG, Walter KA. 5-Fluorouracil for the treatment of intraepithelial neoplasia of the conjunctiva and cornea. Ophthalmology. 2000;107:2190–5.
Midena E, Angeli CD, Valenti M, de Belvis V, Boccato P. Treatment of conjunctival squamous cell carcinoma with topical 5-fluorouracil. Br J Ophthalmol. 2000;84:268–72.
Al-Barrag A, Al-Shaer M, Al-Matary N, Al-Hamdani M. 5-Fluorouracil for the treatment of intraepithelial neoplasia and squamous cell carcinoma of the conjunctiva, and cornea. Clin Ophthalmol Auckl NZ. 2010;4:801–8.
Nutt RJ, Clements JL, Dean WH. Ocular surface squamous neoplasia in HIV-positive and HIV-negative patients and response to 5-fluorouracil in Angola. Clin Ophthalmol Auckl NZ. 2014;8:2435–40.
Rudkin AK, Muecke JS. Adjuvant 5-fluorouracil in the treatment of localised ocular surface squamous neoplasia. Br J Ophthalmol. 2011;95:947–50.
Lewczuk N, Zdebik A, Bogusławska J. Interferon Alpha 2a and 2b in Ophthalmology: A Review. J Interferon Cytokine Res. 2019;39:259–72.
Maskin SL. Regression of limbal epithelial dysplasia with topical interferon. Arch Ophthalmol. 1994;112:1145.
Decatris M, Santhanam S, O’Byrne K. Potential of Interferon-α in Solid Tumours: Part 1. BioDrugs. 2002;16:261–81.
Galor A, Garg N, Nanji A, Joag M, Nuovo G, Palioura S, et al. Human papilloma virus infection does not predict response to interferon therapy in ocular surface squamous neoplasia. Ophthalmology. 2015;122:2210–5.
Mercado C, Ashkenazy N, Karp C, Wang G, Galor A. Immunosuppression as a possible risk factor for interferon non- response in ocular surface squamous neoplasia. Invest Ophthalmol Vis Sci. 2017;58:3352–3352.
Mata E, Conesa E, Castro M, Martínez L, de Pablo C, González ML. [Conjunctival squamous cell carcinoma: paradoxical response to interferon eyedrops]. Arch Soc Espanola Oftalmol. 2014;89:293–6.
Kim HJ, Shields CL, Shah SU, Kaliki S, Lally SE. Giant ocular surface squamous neoplasia managed with interferon Alpha-2b as immunotherapy or immunoreduction. Ophthalmology. 2012;119:938–44.
Kim SE, Salvi SM. Immunoreduction of ocular surface tumours with intralesional interferon alpha-2a. Eye. 2018;32:460–2.
Galor A, Karp CL, Chhabra S, Barnes S, Alfonso EC. Topical interferon alpha 2b eye-drops for treatment of ocular surface squamous neoplasia: a dose comparison study. Br J Ophthalmol. 2010;94:551–4.
Karp CL, Galor A, Chhabra S, Barnes SD, Alfonso EC. Subconjunctival/perilesional recombinant interferon α2b for ocular surface squamous neoplasia. Ophthalmology. 2010;117:2241–6.
Kaliki S, Singh S, Iram S, Tripuraneni D. Recombinant interferon alpha 2b for ocular surface squamous neoplasia: An efficient and cost-effective treatment modality in Asian Indian patients. Indian J Ophthalmol. 2016;64:702.
Schechter BA, Koreishi AF, Karp CL, Feuer W. Long-term follow-up of conjunctival and corneal intraepithelial neoplasia treated with topical interferon alfa-2b. Ophthalmology. 2008;115:1291–.e1.
Krilis M, Tsang H, Coroneo M. Treatment of conjunctival and corneal epithelial neoplasia with retinoic acid and topical interferon Alfa-2b: long-term follow-up. Ophthalmology. 2012;119:1969–73.
Kaliki S, Sharma A, Vempuluru VS. Interferon Alfa-2b for pigmented ocular surface squamous neoplasia: a report of 8 lesions. Cornea. 2020;40:142–6.
Kusumesh R, Ambastha A, Sinha B, Kumar R. Topical interferon α-2b as a single therapy for primary ocular surface squamous neoplasia: asia-pac. J Ophthalmol. 2015;4:279–82.
Shah SU. Topical interferon Alfa-2b for management of ocular surface squamous neoplasia in 23 cases: outcomes based on american joint committee on cancer classification. Arch Ophthalmol. 2012;130:159.
Shields CL, Kaliki S, Kim HJ, Al-Dahmash S, Shah SU, Lally SE, et al. Interferon for ocular surface squamous neoplasia in 81 cases: outcomes based on the American Joint Committee on Cancer classification. Cornea. 2013;32:248–56.
Nanji AA, Moon CS, Galor A, Sein J, Oellers P, Karp CL. Surgical versus medical treatment of ocular surface squamous neoplasia. Ophthalmology. 2014;121:994–1000.
Shields CL, Kancherla S, Bianciotto CG, Lally SE, Shields JA. Ocular surface squamous neoplasia (squamous cell carcinoma) of the socket: management of extensive tumors with interferon. Ophthal Plast Reconstr Surg. 2011;27:247–50.
Stannard C, Sauerwein W, Maree G, Lecuona K. Radiotherapy for ocular tumours. Eye. 2013;27:119–27.
Murthy R, Gupta H, Krishnatry R, Laskar S. Electron beam radiotherapy for the management of recurrent extensive ocular surface squamous neoplasia with orbital extension. Indian J Ophthalmol. 2015;63:672.
El-Assal KS, Salvi SM, Rundle PA, Mudhar HS, Rennie IG. Treatment of invasive ocular surface squamous neoplasia with proton beam therapy. Eye. 2013;27:1223–4.
Ramonas KM. Successful treatment of intraocularly invasive conjunctival squamous cell carcinoma with proton beam therapy. Arch Ophthalmol. 2006;124:126.
Ndlovu N, Ndarukwa S, Kadzatsa W, Rusakaniko S. Evaluation of the radiotherapy management of ocular surface squamous neoplasia in a high HIV prevalence setting- a retrospective review. Infect Agent Cancer. 2016;11:18.
Lecuona K, Stannard C, Hart G, Rice J, Cook C, Wetter J, et al. The treatment of carcinoma in situ and squamous cell carcinoma of the conjunctiva with fractionated strontium-90 radiation in a population with a high prevalence of HIV. Br J Ophthalmol. 2015;99:1158–61.
Walsh-Conway N, Conway RM. Plaque brachytherapy for the management of ocular surface malignancies with corneoscleral invasion. Clin Exp Ophthalmol. 2009;37:577–83.
Buc D, Pilon F, Donnarieix D, Kemeny J-L, Bacin F, Rigal D. Treatment of conjunctival epithelial tumors: brachytherapy with ruthenium-106. J. Fr. Ophtalmol.2003;26:929–39.
Barbazetto IA, Lee TC, Abramson DH. Treatment of conjunctival squamous cell carcinoma with photodynamic therapy. Am J Ophthalmol. 2004;138:183–9.
Çekiç O, Bardak Y, Kapucuoğlu N. Photodynamic therapy for conjunctival ocular surface squamous neoplasia. J Ocul Pharmacol Ther. 2011;27:205–7.
Finger PT, Chin KJ. Refractory squamous cell carcinoma of the conjunctiva treated with subconjunctival ranibizumab (Lucentis): a two-year study. Ophthal Plast Reconstr Surg. 2012;28:85–89.
Zaki AA, Fouad H, Emera S, Labib H. Subconjunctival anti VEGF for conjunctival intraepithelial and invasive neoplasia. Aust J Basic Appl Sci. 2009;3:3186–9.
Faramarzi A, Feizi S. Subconjunctival bevacizumab injection for ocular surface squamous neoplasia. Cornea. 2013;32:998–1001.
Asena L, Dursun Altınörs D. Topical bevacizumab for the treatment of ocular surface squamous neoplasia. J Ocul Pharmacol Ther. 2015;31:487–90.
Ip MH, Robert George CR, Naing Z, Perlman EM, Rawlinson W, Coroneo MT. Topical cidofovir for treatment-refractory ocular surface squamous neoplasia. Ophthalmology. 2018;125:617–9.
Shepler TR, Prieto VG, Diba R, Neuhaus RW, Shore JW, Esmaeli B. Expression of the epidermal growth factor receptor in conjunctival squamous cell carcinoma. Ophthal Plast Reconstr Surg. 2006;22:113–5.
Yu JJ, Fu P, Pink JJ, Dawson D, Wasman J, Orem J, et al. HPV Infection and EGFR activation/alteration in HIV-infected east african patients with conjunctival carcinoma nicot C (ed). PLoS ONE. 2010;5:e10477.
El-Sawy T, Sabichi AL, Myers JN, Kies MS, William WN, Glisson BS, et al. Epidermal growth factor receptor inhibitors for treatment of orbital squamous cell carcinoma. Arch Ophthalmol. 2012;130:1608.
Esmaeli B, Sagiv O. Targeted biological drugs and immune check point inhibitors for locally advanced or metastatic cancers of the conjunctiva, eyelid, and orbit. Int Ophthalmol Clin. 2019;59:13–26.
Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–67.
Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61.
Acknowledgements
Thank you to the Wits Health Sciences Library for their dedication and support.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
Human Research Ethics Committee of the University of the Witwatersrand, M190729.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hӧllhumer, R., Williams, S. & Michelow, P. Ocular surface squamous neoplasia: management and outcomes. Eye 35, 1562–1573 (2021). https://doi.org/10.1038/s41433-021-01422-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01422-3
This article is cited by
-
Melanocytic ocular surface squamous neoplasia successfully managed with no-touch excisional biopsy, cryotherapy and amniotic membrane transplantation
Eye (2024)
-
Comparison of non-invasive diagnostic modalities for ocular surface squamous neoplasia at a tertiary hospital, South Africa
Eye (2024)
-
Ocular surface squamous neoplasia with orbital tumour extension: risk factors and outcomes
Eye (2023)
-
Medical treatment for ocular surface squamous neoplasia
Eye (2023)
-
Topical pharmacotherapy for ocular surface squamous neoplasia: systematic review and meta-analysis
Scientific Reports (2022)