Abstract
Background
To compare 1-year outcomes between anti-vascular endothelial factor (VEGF) therapy and half-dose photodynamic therapy (PDT) for treatment-naive pachychoroid neovasculopathy (PNV) with subretinal fluid (SRF).
Methods
Consecutive patients with treatment-naive PNV patients with SRF treated with intravitreal anti-VEGF injections or half-dose PDT followed by as-needed schedule with 1-year follow-up were studied retrospectively.
Results
Eighty-two eyes of 82 patients were eligible: 50 eyes underwent anti-VEGF therapy and 32 eyes underwent half-dose PDT. SRF resolved in 41 (82%) of 50 eyes after initial three monthly injections and 31 (96.9%) of 32 eyes 3 months after initial PDT, and 43 (86%) eyes and 30 (94%) eyes 1 year after initial anti-VEGF injection and half-dose PDT, respectively. No significant differences were found in SRF resolution rates 3 months and 1 year after initial treatment between the two treatment groups. Best-corrected visual acuity (BCVA) improved significantly after initial three monthly injections (P = 0.025) and initial PDT (P = 0.022) compared with baseline; the improvements were maintained 1 year after initial treatment in the two treatment groups. No significant differences were found in BCVA between the two treatment groups at baseline and throughout the 1-year follow-up period. Mean (± standard error) numbers of intravitreal injections and PDT over 12 months were 3.7 ± 0.16 and 1.1 ± 0.06, respectively.
Conclusions
Both treatments are similarly effective on SRF resolution and VA improvement 1 year after the initial treatment. Half-dose PDT may be an option for treatment for PNV. Prospective studies are required to confirm these findings.
Similar content being viewed by others
Introduction
Pachychoroid neovasculopathy (PNV) is a newly recognized clinical entity of choroidal neovascularization (CNV), i.e., type 1 CNV associated with choroidal thickening and/or dilated Haller’s vessels with a relative absence of drusen. PNV is considered a late complication of pachychoroid pigment epitheliopathy (PPE) and chronic central serous chorioretinopathy (CSC) [1]. The neovascularization in PNV suggests that its etiology differs from typical neovascular age-related macular degeneration (AMD), which may have important management implications. In PNV, focal retinal pigment epithelium (RPE) disturbances and inner choroid attenuation overlying the pachyvessels in PPE and CSC [2,3,4] or chronic inflammation [5] involving the choriocapillaris (CC) may play a role in angiogenesis. PNV is considered a favorable reaction to anti-vascular endothelial growth factor (VEGF) therapy with a longer retreatment-free interval than in more typical neovascular AMD after initial loading injections [6, 7]. However, some eyes with PNV appear refractory to intravitreal anti-VEGF monotherapy, and anti-VEGF therapy in combination with photodynamic therapy (PDT) appears useful for achieving complete subretinal fluid (SRF) absorption and visual improvement after combined therapy [8].
PDT, used widely to treat neovascular AMD before introduction of the anti-VEGF drugs [9], selectively up-takes a photosensitizer in neovascular endothelial cells and selectively ablates CNV through a photochemical reaction [10, 11]. Although nonselective transient vascular occlusion and reduced CC perfusion may occur after PDT [11, 12], it remains a viable treatment option for pachychoroid spectrum diseases such as CSC and polypoidal choroidal vasculopathy (PCV) [12,13,14,15,16,17,18,19]. In CSC, a non-neovascular disease, PDT addresses SRF by inducing hypoperfusion of the hyperpermeable choroidal vessels and decreasing extravascular leakage [19]. Considering this and the different anti-VEGF profiles in patients with PNV from typical neovascular AMD [20, 21], PDT may be beneficial for treating PNV in the pachychoroidal spectrum diseases. Pang and Freund [1] reported two patients with PNV treated with PDT, and Lee and Lee [8] reported that adjunctive PDT treatment in eyes with PNV refractory to anti-VEGF monotherapy resulted in complete fluid absorption in most eyes and visual improvement up to 1 year.
Using half-dose instead of standard-dose PDT has gained in popularity recently for treating chronic CSC because of its similar efficacy and reduced risk of complications [7,8,9]. In the current study, we compared the 1-year outcomes between anti-VEGF therapy and half-dose PDT for treatment-naive PNV.
Materials and methods
This was a retrospective study conducted in a Japanese institutional setting. The Institutional Review Board of the Sapporo Medical Association (Sapporo, Japan) approved the study (serial number r36), which followed the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act regulations; all patients provided written informed consent.
We retrospectively reviewed the charts of treatment-naive patients with symptomatic PNV treated with intravitreal injections of anti-VEGF agents or half-dose PDT at the Hikichi Eye Clinic from May 2, 2016 to May 31, 2019. Treatment allocation was depending on the patients’ decision, which was chose from considerations such as schedule of visiting the clinic and treatment, financial burden, and risk of complications, after enough discussion. The inclusion criteria for this study were the presence of symptoms such as blurred or decreased vision, distorted central vision, central scotoma, metamorphopsia, or micropsia; the presence of subfoveal type 1 CNV detected on spectral-domain optical coherence tomography (OCT) angiography (OCTA) associated with SRF on spectral-domain OCT; and a minimal follow-up period of 12 months after the initial injection of anti-VEGF agents or the initial half-dose PDT.
PNV was diagnosed if all of the following criteria were met [6,7,8, 22]: (1) CNV in either eye; (2) subfoveal choroidal thickness (SFCT) ≥ 200 μm in both eyes; (3) no drusen or only nonextensive (total area, 125 μm circle) hard drusen (≤63 μm) in both eyes (category 1 in the Age-Related Eye Disease Study [23]); (4) CSC or PPE characteristics; namely, choroidal vascular hyperpermeability, RPE abnormality independent of a CNV lesion, the presence of dilated choroidal vessels or choroidal thickening below the type 1 CNV, or a history of CSC. The exclusion criteria were the presence of polyps on indocyanine green angiography (ICGA); and the presence of RPE tears or subretinal fibrosis or any other ocular disease that may have affected the visual acuity (VA). For each patient, only the eye with the longer history of treatment for PNV was included in this study.
At baseline and each follow-up visit, all patients underwent comprehensive ocular examinations including measurement of the best-corrected VA (BCVA) using a Landolt ring chart, OCT, and OCTA. Fluorescein angiography (FA) and ICGA were performed at baseline in all subjects to determine the source of leakage from the PNV and distinguish from PNV to PCV, respectively, and each follow-up visit as needed on a patient-by-patient basis.
The treatment schedule for patients treated with intravitreal injection of ant-VEGF agents (0.5 mg ranibizumab, Lucentis, Genentech, South San Francisco, CA, or 2.0 mg aflibercept (Eylea, Regeneron, Tarrytown, NY) included initial monthly injections for 3 months followed by a reinjection schedule based on need if any of the following occurred [24]: VA loss of at least one line with OCT evidence of fluid at the macula, any qualitative change in the appearance of the OCT images suggesting recurrent fluid in the macula, new macular hemorrhages, or persistent fluid on OCT images 1 month after the previous injection. All criteria were based on comparisons with the previous month’s examination. If any criterion for reinjection was met, the intravitreal injection was administered. Each patient was scheduled for monthly follow-up examinations.
The patients treated with half-dose PDT received 3 mg of verteporfin (Visudyne, Novartis, Basel, Switzerland)/square meter of body surface area, which is half the standard protocol [9], intravenously for 10 min. Fifteen minutes after the infusion started, standard-fluence PDT (light dose, 50 J/cm2; dose rate, 600 mW/cm2; wavelength, 689 nm) was applied for 83 s. The laser spot size was determined by a 1-mm safety margin added to the greatest linear dimension of the CNV on OCTA images. Each patient was scheduled for follow-up examinations at months 1 and 3 after the initial PDT and thereafter at 1- to 3-month intervals based on disease activity. Retreatment criteria for PDT were persistent SRF three months after PDT or recurrent SRF with worsening of symptoms associated with VA loss of at least one line.
The Cirrus 5000 with Angioplex software (Carl Zeiss Meditec, Dublin, CA) [25, 26] was used to obtain the OCT and OCTA images. The real-time tracking software, FastTrac, which is used to minimize eye motion artifacts, achieves sufficient quality 6 × 6-mm angiography images. Two readers (NK and MY) assessed the scan quality (i.e., segmentation errors, motion artifacts, and signal strengths), and low-quality images were excluded. En-face images of the superficial retinal capillary plexus (SCP) layer, deep retinal capillary plexus (DCP) layer, and CC were displayed separately, and the software automatically reported vessel “length” density in mm/mm2 and the area of the foveal avascular zone (FAZ).
Automatic segmentation was performed by the viewing software to generate en-face projection images of CNV lesions after adjusting the level of the segmented layer on the B-scans to best visualize the CNV. Two horizontal segmentation lines contoured to the RPE profile were used to ensure clear visualization of the CNV deep in the RPE [27]. To evaluate the efficacy of the two treatments on the type 1 CNV, the measurement values of CNV areas were compared at baseline and throughout 1-year follow-up period. Type 1 CNV areas on the OCTA images at baseline and each follow-up visit were measured according to a previous report [28]. The same two readers who were masked to the clinical information measured the CNV area on the same slab and manually outlined each CNV area using free image analysis software (ImageJ 1.52a, National Institutes of Health, Bethesda, MD). The pixel counts from the outlined areas were measured using the ImageJ software and converted into area measurements. The square root transformation was used in the analyses. The average of two measurement values performed by the two readers was adopted for the analysis.
The central foveal thickness (CFT) and SFCT, determined based on the average of the vertical and horizontal scans of enhanced-depth-imaging OCT, were measured manually [29]. The CFT and SFCT were defined, respectively, as extending from the inner retinal surface to the RPE line and as the vertical distance between the hyperreflective line of Bruch’s membrane and the chorioscleral interface. In eyes with a subfoveal pigment epithelial detachment (PED), the CFT was defined as the length of the line from the inner foveal surface to the point at a right angle to the line between the edges of the elevated RPE. The two examiners (NK and MY) independently performed the measurements. The average of the measurement values from the two readers was adopted for the analyses.
The main outcome measurement is the rate of SRF resolution defined as complete absence of SRF on OCT image 1 year after the initial treatment, which was compared between the two treatment groups. The secondary outcome measurements such as changes of BCVA, CFT, SFCT, vessel length densities, FAZ area, and CNV area, during 1-year follow-up period were compared between the two treatment groups. Statistical analyses were performed using the EZR software (Saitama Medical Centre, Jichi Medical University, Saitama, Japan). The values between the baseline and various follow-up periods and treatment groups were compared using the Williams test to consider multiplicity issues. The chi-square test or Fisher’s exact test was used to compare two variables in a contingency table to see if they are related. The BCVA was converted to the logarithm of the minimum angle of resolution for statistical purposes. P values less than 0.05 were considered significant.
Results
Eighty-eight eyes of 88 treatment-naive patients with symptomatic PNV were treated during the period, and six patients were lost to follow-up before 1-year after the initial treatment. Thus, 82 eyes of 82 patients were eligible for this study: 50 eyes of 50 patients underwent anti-VEGF therapy (16 women, 34 men; mean ± standard error [SE] age, 64 ± 1.3 years; range, 50–76 years) and 32 eyes of 32 patients underwent half-dose PDT (12 women, 20 men; mean ± SE age, 65 ± 1.9 years; range, 51–75 years). Among the 50 eyes treated with intravitreal injections of anti-VEGF agents, aflibercept was administrated in 34 (68%) eyes and ranibizumab in 16 (32%) eyes. Of 82 eyes, 70 (85%) eyes had subfoveal CNV and 12 (15%) eyes had juxtafoveal CNV defined as the near edge of the CNV is 1–199 µm from the center of the fovea. Table 1 shows the baseline characteristics of the patients who underwent each treatment. No significant differences were seen in any values between the anti-VEGF and half-dose PDT groups.
Forty-one (82%) of 50 eyes treated with anti-VEGF therapy had complete resolution of the SRF on OCT images after initial three monthly injections. Of those 41 eyes, seven (17%) eyes had recurrent SRF during the 1-year follow-up period and were treated with the same anti-VEGF agent initially used. In nine (18%) eyes in which the SRF remained after the initial three monthly injections, anti-VEGF treatment was continued throughout the 1-year follow-up period. Complete resolution of the SRF 1 year after the initial ant-VEGF injection was obtained in 43 (86%) eyes. The mean (± SE) number of intravitreal injections until 12 months were 3.7 ± 0.16.
In 32 eyes treated with half-dose PDT, the SRF resolved in 27 (84%) and 31 (96.9%) eyes on OCT images 1 and 3 months after the initial PDT treatment, respectively. Of the 31 eyes with complete resolution of the SRF 3 months after treatment, the SRF recurred in one eye and two eyes 9 months and 1 year after the initial PDT treatment, respectively. Those three eyes and the one eye with persistent SRF 3 months after the initial PDT treatment underwent a second treatment with half-dose PDT, which achieved complete resolution of the SRF 1 month after the second treatment that was maintained. Complete resolution of the SRF 1 year after the initial half-dose PDT was obtained in 30 (94%) eyes. No significant differences were found in the rate of complete resolution of the SRF between anti-VEGF therapy and half-dose PDT groups (P = 0.463). The mean (± SE) number of PDT applications up to 12 months was 1.1 ± 0.06.
The changes in the mean (± SE) BCVA during the 1-year period are shown in Fig. 1A. Significant improvement was seen in BCVA 1 month after the initial intravitreal injection of anti-VEGF agent (P = 0.025) compared with baseline. The improved BCVA tended to continue to 6 months after the initial injection and the improved BCVA to be maintained 1 year after the first injection (P = 0.018). In eyes treated with half-dose PDT, although no significant improvement was seen in the mean BCVA 1 month after the initial PDT, the BCVA improved significantly (P = 0.022) 3 months after the initial PDT treatment and the improved BCVA was maintained 1 year after the initial PDT (P = 0.027). No significant differences were found in BCVA between the two treatment groups at baseline and throughout the 1-year follow-up period.
A The serial changes in the mean ± standard error (SE) best-corrected visual acuity during 1 year after the initial intravitreal injection of anti-vascular endothelial growth factor (VEGF) agent and the initial half-dose photodynamic therapy (PDT). The solid line represents the anti-VEGF therapy and the dotted line represents half-dose PDT. logMAR logarithm of the minimum angle of resolution. B The serial changes in the mean ± SE central foveal thickness during 1 year after the initial intravitreal injection of anti-VEGF agent and the initial half-dose PDT. The solid line represents the anti-VEGF therapy and the dotted line represents half-dose PDT. C The serial changes in the mean ± SE subfoveal choroidal thickness during the 1 year after the initial intravitreal injection of anti-VEGF agent and the initial half-dose PDT. The solid line represents the anti-VEGF therapy and the dotted line represents half-dose PDT. D The serial changes in the mean ± SE square root of the area of choroidal neovascularization during the 1 year after the initial intravitreal injection of anti-VEGF agent and the initial half-dose PDT. The solid line represents the anti-VEGF therapy and the dotted line represents half-dose PDT.
Figure 1 B shows the changes in the CFT during the 1-year period. The CFT decreased significantly (P = 0.001 for each comparison) 1 month after the initial intravitreal injection of anti-VEGF agents and the initial PDT application compared with baseline, and the reduced CFT was maintained 1 year after the initial treatment (P = 0.010 in anti-VEGF therapy and P = 0.028 in PDT). No significant differences were found in CFT between the two treatment groups at baseline and throughout the 1-year follow-up period.
Regarding the SFCT, a mean reduction from 10 to 20 microns was observed after the initial three monthly injections of the anti-VEGF drug, which did not reach significance compared with the baseline (Fig. 1C). In contrast, a tendency was seen toward thinning of the SFCT 1 month after the initial PDT treatment (320 ± 12 μm) compared with baseline (368 ± 14 μm) (P = 0.050); thereafter, the reductions were significant (P = 0.001 and P = 0.005, respectively) at 3 months and 1 year after the initial PDT treatment. SFCT 1 year after the initial PDT treatment was significantly thinner (P = 0.003) compared with SFCT 1 year after the initial injection of the anti-VEGF drug.
The changes in the FAZ area during the 1-year follow-up period are in Table 2. No apparent changes were found throughout 1 year after the initial treatments in the two treatments. The densities of the vessel lengths in the SCP, DCP, and CC measurements during the 1-year period in Table 2 showed no apparent changes throughout 1 year after the initial treatment among the three variables in the two treatments. The square root of the type 1 CNV area tended to decrease 3 months after both initial treatments compared to baseline, but the difference did not reach significance (P = 0.054 in the anti-VEGF group and P = 0.062 in the PDT group) (Fig. 1D) and thereafter returned to baseline. No significant differences were found between the two treatments at baseline (P = 0.074), and 3 months and 1 year after both initial treatments (P = 0.091), and (P = 0.538), respectively. The good reproducibilities of the inter- and intra-observer agreements in the measurements of the CFT, SFCT, and CNV size had been confirmed previously using Cohen’s kappa coefficient [28, 29].
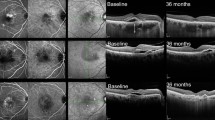
Figure 2 shows the multimodal images at baseline (A, B, C, and D) and 1 month after the initial three monthly injections of aflibercept (E and F) in the left eye of a 62-year-old man. The baseline decimal BCVA was 0.6 (equivalent to 20/32 in Snellen equivalent) and improved to 1.0 (equivalent to 20/20 in Snellen equivalent) 1 month after the initial three monthly injections. At baseline, an infrared image (A) showed scattered macular multifocal granular RPE degeneration, and OCT (B) showed SRF involving the fovea and a thickened choroid. The late-phase simultaneous FA and ICGA scans showed lesions with subretinal hyperfluorescence on FA (C—left) and a subfoveal plaque lesion and choroidal vascular hyperpermeability on ICGA (C—right). Subfoveal type 1 CNV was detected on OCTA en-face images (6 × 6 mm) and the root square of the CNV size was 1.71 mm (D). The SRF resolved 1 month after the initial three monthly injections (E) and the root square of the CNV size slightly decreased (1.66 mm) (F). The baseline CFT (318 μm) decreased apparently to 173 μm 1 month after the initial three monthly injections and the SFCT decreased slightly from 404 to 378 μm.
The baseline infrared image shows scattered macular multifocal granular retinal pigment epithelium degeneration (A); optical coherence tomography (OCT) shows subretinal fluid (SRF) involving the fovea and a thickened choroid (B). The late-phase simultaneous fluorescein angiography (FA) (C—left) and indocyanine green angiography (ICGA) (C—right) scans show lesions with subretinal hyperfluorescence on FA and a subfoveal plaque lesion and choroidal vascular hyperpermeability on ICGA. Subfoveal type 1 choroidal neovascularization (CNV) is seen on OCT angiography en-face images (6 × 6 mm) and the root square of the CNV size is 1.71 mm (D). The SRF resolved 1 month after the initial three monthly injections (E), and the root square of the CNV size (outlined area) was a slight reduced to 1.66 mm (F).
Figure 3 shows the findings at baseline (A and B) and 3 months after the initial half-dose PDT treatment (C and D) on OCT images and OCTA en-face images (6 × 6 mm) of type 1 CNV in the left eye of a 65-year-old man. The baseline decimal BCVA was 0.8 (equivalent to 20/25 in Snellen equivalent) and improved to 1.0 (equivalent to 20/20 in Snellen equivalent) 3 months after treatment. At baseline, OCT (A) showed SRF involving the fovea, a thickened choroid, and a PED. The CFT, SFCT, and root square of the CNV size (B) were 334, 356, and 1.58 mm, respectively. The SRF resolved completely 3 months after PDT treatment (C). The CFT and SFCT decreased to 197 and 282 μm, respectively. The root square of the CNV size slightly decreased to 1.50 mm (D).
At baseline, OCT shows subretinal fluid (SRF) involving the fovea, a thickened choroid, dilated choroidal vessels, and a pigment epithelial detachment (PED) (A). The location of the CNV on the OCTA image (outlined area) corresponds to the PED on the OCT image (B). The root square of the CNV size is 1.58 mm. An OCT image obtained 3 months after PDT treatment shows complete resolution of the SRF, and the central foveal thickness and subfoveal choroidal thickness decreased (C). An OCTA image obtained 3 months after PDT treatment shows a slight reduction of the CNV size (outlined area), the root square size of which is 1.50 mm (D).
Discussion
The current study showed that both treatments successfully controlled the SRF and improved the BCVA in PNV. Considering the current results, the following findings may emerge: both treatments can achieve a similar BCVA improvement, except for the possibility of more rapid improvement with eyes treated with anti-VEGF therapy; both the initial three monthly injections of anti-VEGF agents and the initial half-dose PDT application can obtain favorable outcomes in a dry macula and prevent recurrent exudation for 1 year; both treatments can significantly reduce the CFT, whereas a significant reduction of SFCT can occur in eyes treated with half-dose PDT but not in eyes treated with anti-VEGF therapy; any apparent deteriorations of vessel densities of the retinal capillary plexuses or CC are not induced by either treatment; and the sizes of the type 1 CNV area after both the initial three monthly injections of anti-VEGF agents and the initial half-dose PDT tended to decrease and thereafter returned to baseline.
Although the etiology of development of type 1 CNV in PNV remains elusive, it is suggested to differ compared to typical neovascular AMD, which may have important management implications. The neovascularization may be triggered by focal RPE disturbances and inner choroid attenuation overlying pachyvessels [2,3,4], which may lead to upregulation of VEGF. Others have proposed that chronic inflammation involving the CC also may play a role in angiogenesis [5]. Hata et al. [20] reported lower intraocular VEGF levels in PNV than in neovascular AMD. Nevertheless, the authors reported that PNV responded favorably to anti-VEGF therapy. Matsumoto et al. [7] and Jung et al. [6] also reported the efficacy of anti-VEGF therapy on PNV. Padrón-Pérez et al. [30] suggested that the mechanism of action underlying the decreased choroidal thickness after anti-VEGF therapy may be reduced abnormal hyperpermeability of the choroidal vessels, the diameter of the dilated choroidal vessels, and leakage from the PNV.
Jung et al. [6] reported that full-dose PDT was applied in six eyes that did not respond to the initial three monthly intravitreal anti-VEGF injections, and the maculae were dry 3 months after PDT treatment in all eyes without recurrent fluid accumulation by the end of the 1-year follow-up period. Lee and Lee [8] also reported that adjunctive PDT in eyes with PNV refractory to anti-VEGF monotherapy resulted in complete fluid absorption in most eyes and visual improvement up to 1 year. Those authors stated that higher ocular perfusion pressure may cause more vigorous exudation from PNV, which may overcome the anti-permeability action of the anti-VEGF agents. Another possibility is that increased hydrostatic pressure in the choroid results in additional exudation as in CSC, which is addressed inadequately with anti-VEGF agents. The PDT-induced choroidal hypoperfusion, which significantly reduced the choroidal thickness [8], may be advantageous for resolving exudates.
PDT has been reported to be associated with a possible risk for damage to normal choroidal vasculature and RPE [14, 31]. Reibaldi et al. [15] evaluated the efficacy of low-fluence compared with standard-fluence PDT for treating chronic CSC and found that the treatment-induced CC hypoperfusion was less with low-fluence PDT than with standard-fluence PDT, as seen on early and late ICGA images. A study that compared the efficacy and safety between half-fluence and half-dose PDT in chronic CSC reported that half-dose PDT induced more rapid fluid reabsorption, a longer lasting effect, and equal safety compared with half-fluence PDT [16]. Thus, we chose to use half-dose PDT in the current study. The current results showed that the vessel densities in the SCP, DCP, CC, and the FAZ area measurements remained unchanged 1 year after application of half-dose PDT for PNV. Similar to treatment with half-dose PDT for CSC, half-dose PDT for PNV may minimally affect the retinal capillary plexuses and CC.
The retrospective study conducted by Jung et al. [6] compared the efficacy of intravitreal injections of ranibizumab and aflibercept for 54 eyes with PNV and found that aflibercept was more effective in absorbing SRF in the short term. The reduction in the SFCT was significantly higher in the aflibercept group without a significant difference in the visual outcomes between the two groups after three initial injections. In the current study, no apparent differences were seen in the visual outcomes or reductions in SFCT between the two groups throughout the 1-year follow-up period, which may have resulted from the small sample size.
The limitations of the current study included its retrospective and non-randomized comparative nature and the relatively few subjects. Second, the current study did not compare treatment outcomes between standard PDT and half-dose PDT. Standard PDT may obstruct the CNV and CC, findings that were not apparent after half-dose PDT in the current study. Third, the recently developed OCTA has some limitations, in that artifacts present in OCTA images remain important issues in the technology [32]. Despite the limitations, the current results showed the similar efficacy of anti-VEGF therapy and half-dose PDT on the rate of complete resolution of SRF and VA improvement 1 year after the initial treatment. Half-dose PDT required fewer additional treatments for PNV during a 1-year follow-up period. Our results suggested that half-dose PDT may be an option for treatment for PNV. Further prospective and comparative studies are needed to determine the best treatment for PNV.
Summary
What was known before
-
PNV is a newly recognized clinical entity of CNV.
-
Etiology of neovascularization in PNV is suggested to differ from typical AMD.
-
Some eyes with PNV appear refractory to intravitreal anti-VEGF monotherapy.
What this study adds
-
Anti-VEGF therapy and half-dose PDT successfully can control the SRF and improved the BCVA in PNV.
-
Half-dose PDT may be an option for treatment for PNV.
References
Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015;35:1–9.
Peiretti E, Ferrara DC, Caminiti G, Mura M, Hughes J. Choroidal neovascularization in Caucasian patients with longstanding central serous chorioretinopathy. Retina. 2015;35:1360–7.
Dansingani KK, Balaratnasingam C, Naysan J, Freund KB. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina. 2016;36:499–516.
Lee WK, Baek J, Dansingani KK, Lee JH, Freund KB. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or subnormal subfoveal choroidal thickness. Retina. 2016;36:S73–S82.
Kitaya N, Nagaoka T, Hikichi T, Sugawara R, Fukui K, Ishiko S, et al. Features of abnormal choroidal circulation in central serous chorioretinopathy. Br J Ophthalmol. 2003;87:709–12.
Jung BJ, Kim JY, Lee JH, Baek J, Lee K, Lee WK. Intravitreal aflibercept and ranibizumab for pachychoroid neovasculopathy. Sci Rep. 2019;2055. https://doi.org/10.1038/s41598-019-38504-y.
Matsumoto H, Hiroe T, Morimoto M, Mimura K, Ito A, Akiyama H. Efficacy of treat-and-extend regimen with aflibercept for pachychoroid neovasculopathy and type 1 neovascular age-related macular degeneration. Jpn J Ophthalmol. 2018;62:144–50.
Lee JH, Lee WK. One-year results of adjunctive photodynamic therapy for type 1 neovascularization associated with thickened choroid. Retina. 2016;36:889–95.
Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials-TAP report. Arch Ophthalmol. 1999;117:1329–45.
Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age- related macular degeneration. Surv Ophthalmol. 2000;45:195–214.
Schmidt-Erfurth U, Michels S, Barbazetto I, Laqua H. Photodynamic effects on choroidal neovascularization and physiological choroid. Investig Ophthalmol Vis Sci. 2002;43:830–41.
Lee PY, Kim KS, Lee WK. Severe choroidal ischemia following photodynamic therapy for pigment epithelial detachment and chronic central serous chorioretinopathy. Jpn J Ophthalmol. 2009;53:52–6.
Koh A, Lai TYY, Takahashi K, Wong TY, Chen LJ, Ruamviboonsuk P, et al. EVEREST II study group. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol. 2017;135:1206–13.
Van Rijssen TJ, Van Dijk EHC, Scholz P, et al. Focal and diffuse chronic central serous chorioretinopathy treated with half-dose photodynamic therapy or subthreshold micropulse laser: PLACE Trial Report No. 3. Am J Ophthalmol. 2019;205:1–10.
Reibaldi M, Cardascia N, Longo A, Furino C, Avitabile T, Faro S, et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol. 2010;149:307–15. e2.
Nicoló M, Eandi E, Alovisi C, Grignolo FM, Traverso CE, Musetti D, et al. Half-fluence versus half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2014;157:1033–7.
Chan WM, Lam DS, Lai TY, Tam BSM, Liu DTL, Chan CKM. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003;87:1453–8.
Maruko I, Iida T, Oyamada H, Sugano Y, Ojima A, Sekiryu T. Choroidal thickness changes after intravitreal ranibizumab and photodynamic therapy in recurrent polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013;156:548–56.
Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, Spaide RF. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010;117:1792–9.
Hata M, Yamashiro K, Ooto S, Oishi A, Tamura H, Miyata M, et al. Intraocular vascular endothelial growth factor levels in pachychoroid neovasculopathy and neovascular age-related macular degeneration. Investig Ophthalmol Vis Sci. 2017;58:292–8.
Terao N, Koizumi H, Kojima K, Yamagishi T, Yamamoto Y, Yoshii K, et al. Distinct aqueous humour cytokine profiles of patients with pachychoroid neovasculopathy and neovascular age-related macular degeneration. Sci Rep. 2018:10520. https://doi.org/10.1038/s41598-018-28484-w.
Miyake M, Ooto S, Yamashiro K, Takahashi Å, Yoshikawa M, Akagi-Kurashige Y, et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci Rep. 2015;5:16204.
Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132:668–81.
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO study. Am J Ophthalmol. 2009;148:43–58.
Uji A, Balasubramanian S, Lei J, Baghdasaryan E, Al-Sheikh M, Sadda SR. Impact of multiple en face image averaging on quantitative assessment from optical coherence tomography angiography images. Ophthalmology. 2017;124:944–52.
Hikichi T, Agarie M. Reduced vessel density of the choriocapillaris during anti-vascular endothelial growth factor therapy for neovascular age-related macular degeneration. Investig Ophthalmol Vis Sci. 2019;60:1088–95.
Seddon JM, McLeod DS, Bhutto IA, Villalonga MB, Silver RE, Wenick AS, et al. Histopathological insights into choroidal vascular loss in clinically documented cases of age-related macular degeneration. JAMA Ophthalmol. 2016;134:1272–80.
Hikichi T, Agarie M, Kubo N, Yamauchi M. Predictors of recurrent exudation in choroidal neovascularization in age-related macular degeneration during a treatment-free period. Retina. (in press). https://doi.org/10.1097/IAE.0000000000002745.
Hikichi T, Kitamei H, Shioya S, Higuchi M, Matsushita T, Kosaka S, et al. Relation between changes in foveal choroidal thickness and 1-year results of ranibizumab therapy for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2014;98:1201–4.
Padrón-Pérez N, Arias L, Rubio M, Lorenzo D, García-Bru P, Català-Mora J, et al. Changes in choroidal thickness after intravitreal injection of anti-vascular endothelial growth factor in pachychoroid neovasculopathy. Investig Ophthalmol Vis Sci. 2018;59:1119–24.
Dewi NA, Yuzawa M, Toching K, Kawamura A, Mori R. Effects of photodynamic therapy on the choriocapillaris and retinal pigment epithelium in the irradiated area. Jpn J Ophthalmol. 2008;52:277–81.
Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence angiography. Retina. 2015;35:2163–80.
Author information
Authors and Affiliations
Contributions
Conception and design: TH. Analysis and interpretation: TH, NK, MY. Data collection: TH, NK, MY. Overall responsibility: TH, NK, MY.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hikichi, T., Kubo, N. & Yamauchi, M. One-year comparison of anti-vascular endothelial growth factor and half-dose photodynamic therapies for pachychoroid neovasculopathy. Eye 35, 3367–3375 (2021). https://doi.org/10.1038/s41433-021-01418-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01418-z
This article is cited by
-
Pachychoroid neovasculopathy has clinical properties that differ from conventional neovascular age-related macular degeneration
Scientific Reports (2023)
-
Optical coherence tomography-based misdiagnosis and morphological distinction in pachychoroid neovasculopathy vs. polypoidal choroidal vasculopathy
Eye (2023)
-
Predictive factors for outcomes of half-dose photodynamic therapy combined with aflibercept for pachychoroid neovasculopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Vanishing pachy-choroid in pachychoroid neovasculopathy under long-term anti-vascular endothelial growth factor therapy
BMC Ophthalmology (2021)
-
Long-term outcome of intravitreal anti-vascular endothelial growth factor treatment for pachychoroid neovasculopathy
Scientific Reports (2021)