Abstract
Purpose
To assess optical coherence tomography angiography (OCTA) parameters in children born preterm who developed spontaneously regressed retinopathy of prematurity (sr-ROP, group 1), or had no ROP (PreT, group 2), compared with term-born age-matched controls (group 3).
Methods
Cross-sectional comparative case series. Children aged 6–8 years had a complete ocular examination and OCT and OCTA imaging (Optovue RTVue AVANTI instrument). Foveal avascular zone (FAZ) area, FAZ perimetry, and vascular density in three slabs were measured automatically, and foveal depth was measured manually by two graders.
Results
Groups 1, 2, and 3 (26, 32, and 34 eyes respectively) did not differ in age or gender, but differed in gestational age and birth weight. Both inner retinal thickness and foveal depth differed significantly between group 1 and 2, as did vascular density in the superficial and deep vascular plexus. VA, FAZ area, and perimetry were distinct in all three groups. VA correlated positively with FAZ area and foveal depth, negatively with vascular density.
Conclusion
OCTA parameters reveal microvascular changes that distinguish eyes with sr-ROP from premature eyes without ROP, as does visual acuity. It is not possible to infer if the cause is the presence of retinopathy or the different severity of retinal immaturity.
Similar content being viewed by others
Introduction
It is increasingly being recognized that children with a history of retinopathy of prematurity (ROP) that regressed spontaneously or after treatment, but also children born preterm who did not develop ROP, do not have entirely normal retinal microstructure and function [1,2,3,4]. Advancing imaging technology has helped discover these subtle anatomical differences that are otherwise clinically invisible.
Studies so far have described several features of the retina in former preterm children, however it has not been clearly assessed if these changes pertain to prematurity itself, to the development of retinopathy in a preterm, or finally to structural changes subsequent to the applied treatment for ROP. Likewise, it has not been clarified if the magnitude of the changes is associated with the severity of the developed retinopathy in early life, nor if these changes are stable overtime.
Most studies give supporting evidence that premature birth alters the neuronal and angiogenic maturation of the retina not only in the periphery but also at the level of the macula, and the term “macular developmental arrest” has been proposed to describe this [5]. The aim of this study was to assess foveal vascular microstructure in preterm subpopulations which are closer to normal, i.e., address these changes in children born preterm and who either developed self-limiting ROP or did not develop retinopathy at all.
Methods
In this cross-sectional observational comparative case series, all children born preterm and hospitalized in the neonatal intensive care unit of our hospital in 2010–2012 and who had a history of spontaneously regressed retinopathy of prematurity (sr-ROP) were invited to participate (group 1). A second group of children born preterm in the same period and who did not develop any ROP (PreT) were also invited to participate (group 2). Finally, a control group of age-matched full-term children with normal ocular examination was recruited from the pediatric ophthalmology outpatient clinic (group 3). Exclusion criteria were other retinal or developmental diseases, systemic cardiovascular or inflammatory diseases, and history of ocular trauma or surgery. Also, eyes with amblyopia, poor fixation, or children with inadequate cooperation were excluded. Institutional Review Board/Bioethics Committee approved the study. The study adhered to the tenets of the declaration of Helsinki and informed consent was obtained from the parents or guardians of all children.
All children had a complete ocular examination, including visual acuity (VA) measurement, refraction, stereoacuity, oculomotility, and anterior and posterior segment examination. VA was measured in logMAR values with the ETDRS chart at 4 m in an illuminated box. When unaided VA in either eye was worse than 0.0 logMAR, cycloplegic refraction was measured in both eyes with autorefractometry and confirmed by retinoscopy after instillation of one drop of cyclopentolate 1%. Retinal imaging was performed after dilation.
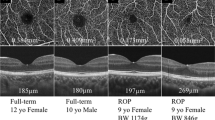
Optical coherence tomography (OCT) and OCT angiography (OCTA) scans of the macula were acquired using the Optovue RTVue AVANTI instrument (Optovue Inc., Fremont, CA). OCT scanning of the macula was performed with the Retina Map protocol. HD Angio Retina OCTA images with a scan size 6 mm × 6 mm were also acquired. The instrument’s AngioVue software module was used to analyse the OCTA images for foveal avascular zone (FAZ) area, FAZ perimetry, and vascular density (VD). VD was quantified in each eye separately for the fovea (central 1 mm area in the ETDRS map), parafovea (1–3 mm), and perifovea (3–6 mm) regions and in three different slabs: the whole retina, the superficial vascular plexus (SVP), and deep vascular plexus (DVP). The upper boundary used for segmenting the SVP slab was 3 μm below the internal limiting membrane and the lower was boundary was 15 μm below the inner plexiform layer. The DVP was segmented between 15 μm below the inner plexiform layer (which was also the lower boundary of the SVP slab) and 70 μm below the inner plexiform layer. To enter analysis, OCTA scans had to meet minimum criteria for image quality including a signal strength index of 40 or more and absence of visible motion artefacts. All images were also inspected for segmentation issues. Both eyes were scanned and entered analysis if they met these criteria. Examples of OCT and OCTA images from participants in each group are shown in Fig. 1.
OCT angiography images from selected patients. Superficial vascular plexus (SVP) and deep vascular plexus (DVP) en face slabs are shown in selected patients from groups 1 (a1, b1), 2 (a2, b2), and 3 (a3, b3 respectively). In c1–c3 the foveal avascular zone (FAZ) area is delineated by the instrument’s automated software in the OCT angiography image in the same patients from groups 1, 2, and 3. In d1–d3, the macula OCT image is graded to quantify the fovea depth in the same patients.
Inner retina, outer retina, and total retina values were recorded from the volume OCT scans for the fovea, parafovea, and perifoveal regions. Foveal depth was measured manually on a horizontal B scan passing through the foveal center. Two graders (MD and CT) graded the de-identified images independently. The instrument’s calliper tool was utilized to measure the lowest vertical distance between the fovea pit and a central 1.5 mm line connecting the highest peaks following the fovea contour.
Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, Il). Means and standard deviations were computed for continuous variables. Comparisons between the three groups were made with one-way ANOVA and post hoc comparisons with the Bonferroni correction were performed to identify significant differences. Agreement between graders in the measurement of foveal depth was assessed with the intraclass correlation coefficient for absolute agreement. Foveal depth data from grader 1 were used in the analysis. Spearman correlation coefficients were computed to analyze for correlations between variables. A p value < 0.05 was considered significant.
Results
Twenty six eyes were included in the sr-ROP group (group 1), 32 eyes in the PreT group (group 2), and 34 in the control group (group 3). In sr-ROP group, five patients were diagnosed with stage 1 ROP, three patients with stage 2 ROP, and five patients with stage 3 ROP, all in anterior zone II or zone III. There was no difference in age and gender between the three groups. As expected, gestational age and birth weight differed between the groups. Participants’ demographics data for each group are presented in Table 1. All participants were Caucasians, of Greek ethnicity.
VA was found to differ significantly between all three groups (lower in sr-ROP than PreT as well as control group, lower in PreT than in control group, Table 1). Cycloplegic refraction (spherical equivalent) in the 16 cyclopleged participants ranged from −3D to +4.5D (median 0.625D, interquartile range +0.68D to +1.75D). Most of the children had normal stereoacuity (40/46 children had positive Lang I test). Two had a convergent squint without strabismic amblyopia, and one child had nystagmus, mild enough to permit imaging of sufficient quality. The rest of ocular examination was unremarkable.
Analysis of OCT thickness values identified differences only in the fovea region (Table 2). No differences were observed in the inner or outer retina thicknesses in the parafovea and perifovea regions between the groups (p > 0.05). Interestingly, the sr-ROP group had increased inner, outer, and total retina thickness in the fovea when compared with the control group. However, significant differences were only observed in foveal inner retina thickness when comparing the sr-ROP and the PreT groups. The PreT group also showed increased outer retina and total retina thickness in the fovea region compared with the control group.
In addition, foveal depth was significantly different in the sr-ROP group compared with either group 2 or 3, while measurements were similar when comparing the PreT and control groups. Therefore, foveal depth was another OCT parameter that differed between sr-ROP and PreT groups. Importantly, the agreement between the two graders in measuring foveal depth on OCT was excellent. Intraclass correlation coefficient for absolute agreement, single measures was 0.972.
OCTA metrics included FAZ area, FAZ perimetry, and VD in three locations and three slabs. Interestingly, FAZ area, FAZ perimetry and fovea VD parameters on OCTA were significantly different in the sr-ROP compared with the other two groups. Both FAZ area and perimetry were smaller in the sr-ROP group compared with groups 2 and 3 (Table 3). Moreover, VD in the fovea region was significantly higher in group 1, compared with groups 2 and 3, when analyzing either the SVP or the DVP (Table 3). In fact, FAZ area, FAZ perimetry, and total retina fovea VD values were significantly different in each group. Comparisons between the PreT group and the control group in OCTA VD in the SVP or the DVP did not reach significance. Similar to OCT, there was no difference in any of the OCTA metrics of the parafovea and perifovea regions.
In addition, we tested for correlations between OCT/OCTA parameters and VA expressed in logMAR values. FAZ area and foveal depth were negatively correlated to VA (ρ = −0.741, p = 0.002 and ρ = −0.303, p = 0.005 respectively), i.e., the bigger the FAZ area the smaller logMAR VA was, meaning better acuity. Foveal thickness on OCT and VD on OCTA showed a statistically significant positive correlation to VA (ρ = 0.279, p < 0.001 and ρ = 0.369, p = 0.001 respectively), i.e., the bigger the foveal thickness the higher logMAR VA was, meaning poorer acuity.
Finally, no correlation was found between ROP stage and foveal depth (p = 0.776), FAZ area (p = 0.585), SVP VD (p = 0.609) or DVP VD (p = 0.966).
Discussion
In this study FAZ area and perimetry, and vascular density both in the superficial and in the deep vascular plexus of the fovea, were found to be distinctly different in previously preterm children with self-limiting ROP and in those without clinically recognized retinopathy, and finally in age-matched term-born children. Our results show that microvascular changes at the fovea, as well as foveal depth, correlate with the level of visual acuity in these three groups of children, potentially reflecting a spectrum of subnormal postnatal development of the macula.
OCT findings showed that group 1 (sr-ROP) and group 2 (PreT) display increased overall retinal thickness compared with controls (group 3) but do not show differences between them. The only parameter than distinguishes them is inner retinal thickness, which is higher in group 1 than in group 2. This is similar to what Bowl et al. [5] found in their 2016 study of three large groups of children similar to ours tested at 6–13 years of age, i.e., GCL + thickness (ganglion cell layer + inner plexiform layer) higher in group 1 than in group 2 or 3, except that in their series total retinal thickness was distinct between the three groups. Wu et al. [1] also looked at groups of treated, sr-ROP, no-ROP, and full-term controls, but only made comparisons between retinal thickness in treated eyes and normal eyes and not between the rest of the groups.
On the other hand, the use of OCTA imaging allows detection of several distinct features between groups 1 and 2, and of course the control group. These features are FAZ area, FAZ perimetry, as well as VD in the fovea region, both SVP and DVP.
The FAZ area and FAZ perimetry were found to be statistically different between groups 1 and 2, as well as different to group 3. This has not been directly shown in the literature so far. Notably, Bowl et al. [6] found a smaller FAZ in a group of children with sr-ROP (similar to our group 1) than in term-born children (as in our group 3), tested at 5 years of age (average). On the other hand, Balasubramanian et al. [3] found a smaller FAZ area in a group of former preterm children compared to term-born children (as in our group 3), but there was no difference in FAZ area within the preterm group, i.e., between those who had a positive or no history of treatment for ROP. In the latter study, the children in each group had a large range of ages, which has been shown to influence the FAZ area [7], so this factor may have confounded the results.
The outer retinal layer shows similar thickness in groups 1 and 2 in our series, as has also been well described in the literature [1, 2]. However, the DVP, which in part corresponds anatomically with the outer retinal layer, shows significant differences between groups 1 and 2. This reflects a difference in the foveal vascular network which may not be picked up in the OCT and is only depicted with OCTA.
One could assume that the difference between groups 1 and 2 in DVP may be the result of artefacts from shadowing of the vascular networks overlying the DVP. However, these artefacts are more prominent in the parafoveal and even more in the perifoveal areas, where larger vessels exist in the superficial layers of the retina. In our series of cases there was no difference of the DVP between groups when looking at the parafoveal and perifoveal areas, and this difference only existed in the fovea, so it must be true.
In our sr-ROP group of children the stage of retinopathy was not found to be correlated with foveal depth, FAZ area or vascular density in the SVP or DVP. This finding is in line with the report by Bowl et al. [5] as far as foveal thickness is concerned. Also, Dubis et al. [8] found that persistence of inner retinal layers in the macula and presence of cystoid macular oedema exists in premature infants with ROP regardless of the maximal ROP stage. Interestingly, a recent study by Anwar et al. [9] suggested that fovea width, rather than fovea depth, may be a new important parameter to assess in infants as an early indicator of ROP development. The correlation with ROP remained significant after adjusting for gestational age and birth weight. Future studies may provide further evidence on potential ROP biomarkers from structural OCT.
An important factor to take into account is the fact that imaging of the retina of our patients was taken at the age of seven and a half years and not in the neonatal period. Children in groups 1 and 2 do differ in birth weight and gestational age. Hence, the depicted differences may be presumed to be due to a more severely immature retinal vasculature at birth in group 1 as opposed to group 2 and control group 3. In other words our results may be reflecting a gradient of severity of immaturity rather than a difference due to the presence of retinopathy. This is a key question that the present study cannot answer but can certainly highlight, given that the retinopathy in our cases was mild and self-limited. Only a few premature infants are reported to have been imaged with OCTA during infancy [8, 10], so no conclusions can so far be drawn as to the timing and profile of vasculature development in the less or more premature infants. Given the cross-sectional design of the current study, it cannot assess if changes between groups 1 and 2 are already present at birth or develop during the neonatal period, neither if they are related to the development of retinopathy or not.
Visual acuity was distinctly different between all three groups, as has also been previously shown [5]. It is arguable that this difference is only statistically and not clinically significant, however recognition acuity with maximal contrast is only a gross assessment of visual function and so a detectable difference is suggestive of a true difference of functional level. Acuity was found to correlate positively with FAZ area and foveal depth, and negatively with VD at the fovea, which is in line with findings by Balasumaranian et al. [3] as opposed to the report by Villegas et al. [2]. In the latter, no correlation was found between foveal depression and VA, however this was a series of more severely affected subjects most of whom had total absence of foveal depression. Nevertheless, FAZ area appears not to be correlated to VA in normal, term-born children [10], so it could be that FAZ area is only a proxy for another parameter or combination of parameters that contribute to the complex function of visual acuity.
A limitation of this study is the relatively small number of cases in each subgroup, which may have contributed to some attempted associations coming up as trends and not reaching statistical significance. Another limitation is the cross-sectional design. It will be of interest to follow-up these subpopulations of children in order to see how these anatomical markers develop overtime. On the other hand, in our study, the represented groups were carefully chosen to distinguish separate subpopulations rather than to classify them together, so as to reveal potential differences between them. Previous studies have for example grouped together children who received different treatment modalities, or children with a positive or negative history of retinopathy. Another strength is the homogeneity of subpopulations in terms of age, refractive error, male to female ratio, and ethnicity, factors which may account for variation in the anatomic variables assessed [7, 11]. Lastly, a strength of the study is that all included premature children came from a cohort with documented retinopathy status at the time of screening for ROP, in the same setting and by the same examiner (AM).
OCTA is a noninvasive way of depicting subtle subclinical characteristics of the retinal vasculature but there is still a lot to learn in order to correctly interpret these images we obtain and attempt associations. Notably the observed range of values in published studies has been large so it is important to investigate as homogeneous subpopulations as possible. Age at examination is an important factor not to be overlooked, or incorrect interpretations may result [7]. Longitudinal studies should provide a better understanding of the evolution of the parameters depicted with OCTA imaging in the particular subpopulations of children born preterm.
Summary statement
OCT angiography reveals that FAZ area and vascular density are distinct in eyes from premature children with spontaneously regressed ROP and premature children without ROP, and so is visual acuity. This difference may result from the presence of retinopathy, or alternatively from the different severity of immaturity.
Summary
What was known before
-
Macular microstructure is subnormal in children with a history of prematurity with or without ROP.
What this study adds
-
The macular vascular network of children with spontaneously regressed OCT angiography presents increased VD and smaller FAZ area and perimetry, compared with premature children without retinopathy and to term-born controls.
References
Wu WC, Lin RI, Shih CP, Wang NK, Chen YP, Chao AN, et al. Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology. 2012;119:1907–16.
Villegas VM, Capo H, Cavuoto K, McKeown CA, Berrocal AM. Foveal structure-function correlation in children with history of retinopathy of prematurity. Am J Ophthalmol. 2014;158:508–12.
Balasubramanian S, Borrelli E, Lonngi M, Velez F, Sarraf D, Sadda SR, et al. Visual function and optical coherence tomography angiography features in children born preterm. Retina. 2018;00:1–7.
Falavaryani KG, Iafe NA, Velz FG, Schwartz SD, Sadda SR, Sarraf D, et al. Optical coherence tomography angiography of the fovea in children born preterm. Retina. 2017;37:2289–94.
Bowl W, Stieger K, Bokun M, Schweinfurth S, Holve K, Andrassi-Darida M, et al. OCT-based macular structure-function correlation in dependence on birth weight and gestational age—the Giessen long-term ROP study. Investig Ophthalmol Vis Sci. 2016;57:OCT235–41.
Bowl W, Bowl M, Schweinfurth, Holve K, Knobloch R, Stieger K, et al. OCT angiography in young children with a history of retinopathy of prematurity. Ophthalmol Retina. 2018;0:1–7.
Borelli E, Lonngi M, Balasubramanian S, Tepelus TC, Baghdasaryan E, Iafe NA, et al. Macular microvascular networks in healthy pediatric subjects. Retina. 2018;0:1–9.
Dubis AM, Subramaniam D, Godara P, Carroll J, Costakos DM. Subclinical macular findings in infants screened for retinopathy of prematurity with spectral-domain optical coherence tomography. Ophthalmology. 2013;120:1665–71.
Anwar S, Nath M, Patel A, Lee H, Brown S, Gottlob I, et al. Potential utility of foveal morphology in preterm infants measured using hand-held optical coherence tomography in retinopathy of prematurity screening. Retina. 2019. https://doi.org/10.1097/IAE.0000000000002622.
Campbell JP, Nudleman E, Yang J, Tan O, Chan RVP, Chiang MF, et al. Handheld optical coherence tomography angiography and ultra-wide-field coherence tomography in retinopathy of prematurity. JAMA Ophthalmol. 2017;135:977–81.
Cheung CY, Li Y, Yuan N, Lau GYL, Chan AYF, Lam A, et al. Quantitative retinal microvasculature in children using swept-source optical coherence tomography: the Hong Kong children eye study. Br J Ophthalmol. 2018;bjophthalmol-2018-312413.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mataftsi, A., Dermenoudi, M., Dastiridou, A. et al. Optical coherence tomography angiography in children with spontaneously regressed retinopathy of prematurity. Eye 35, 1411–1417 (2021). https://doi.org/10.1038/s41433-020-1059-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-1059-x
This article is cited by
-
Retinal and peripapillary vascular deformations in prematurely born children aged 4–12 years with a history of retinopathy of prematurity
Scientific Reports (2023)
-
Evaluation of macular microvascular structure with optical coherence tomography angiography in children with history of treatment for retinopathy of prematurity
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)