Abstract
Objectives
The objective of this paper is to evaluate visual acuity (VA) outcomes of intravitreal anti-vascular endothelial growth factor (VEGF) in diabetic macular oedema (DMO).
Methods
In this retrospective cohort study, electronic medical records for all patients undergoing intravitreal injections in a tertiary referral centre between March 2013 and October 2018 were analysed. Treatment response in terms of VA outcomes was reported for all eyes over a 4-year observation period.
Results
Our cohort includes 2614 DMO eyes of 1964 patients over 48 months. Cox proportional-hazards modelling identified injection number (hazard ratio (HR) = 1.18), male gender (HR = 1.13) and baseline VA (HR = 1.09) as independent predictors to reach a favourable visual outcome of more than 70 Early Treatment Diabetic Retinopathy Study letters. Half of our cohort reached 70 letters 1.9 months after starting anti-VEGF therapy. Of those that reached 70 letters, 50% fell below 70 letters by 14.7 months.
Conclusion
To date, this is the largest single centre cohort study and over the longest observation period reporting on real-life outcomes of anti-VEGF in DMO. We have made an anonymised version of our data set available on an open-source data repository as a resource for clinical researchers globally.
Similar content being viewed by others
Introduction
There are currently 21 million patients with diabetic retinopathy worldwide, which is expected to increase with the projected prevalence of diabetes mellitus (DM) from 415 million in 2015 to 642 million in 2040 [1]. The overall risk of diabetic macular oedema (DMO) in patients with DM is currently estimated at 7% (and at 29% after 20 years of disease duration), thus establishing it as the major cause for moderate vision loss in diabetic patients [2]. Randomised controlled trials (RCTs) have demonstrated that intravitreal injections (IVI) with anti-vascular endothelial growth factor (VEGF) agents improve the prognosis of patients with DMO in terms of visual acuity (VA) when following a fixed intervals treatment regimen [3,4,5,6].
In generating the evidence base for optimal patient management, analyses of real-world clinical data have become complements to clinical trials. Evaluation of real-world outcomes ensures continued endorsement of therapeutics by regulators, payers, clinicians and patients. Compared with clinical trials, real-world studies typically feature a larger sample size and with it, greater heterogeneity amongst their patient cohort and healthcare delivery systems. Such data enable holistic understanding of a therapeutic as they include a more accurate representation of the patient cohorts that receive it, how it is used, and the resultant outcomes. Notably, treatment conditions in the pivotal anti-VEGF in DMO RCTs are not reflected in real-life clinical settings. A key example is that real-life practice features lower injection frequencies when compared to RCTs [7,8,9,10,11,12,13,14,15,16]. This is likely due to different treatment regimens (pro re nata and treat-and-extend) and reduced therapy adherence. Consequently, visual outcome of daily clinical practice remains unclear.
Here we report on the largest retrospective cohort of patients with DMO (2614 eyes of 1964 patients) receiving anti-VEGF therapy and over the longest observation period (48 months) to date. Time-to-event analyses and Cox proportional-hazards modelling were carried out to evaluate key positive- (reaching VA of 70 Early Treatment Diabetic Retinopathy Study (ETDRS) letters or greater and remaining above 70) and negative visual outcomes (VA loss ≥ 15). An anonymised version of this data set and our analyses will be made available in an open-source digital repository to increase transparency andaccessibility, and to permit independent replication of our results. Such availability also enables our data to contribute toward top tier evidence and greater clinical impact.
Methods
Study setting and design
This study is a retrospective cohort study of diabetic patients treated for DMO by anti-VEGF at a tertiary referral centre—Moorfields Eye Hospital NHS Foundation Trust, London, UK. Approval for the study was provided by the clinical audit committee of the hospital (MEH-233) as well as the local research ethics committee (ROAD17/031). In this study, we complied with the Declaration of Helsinki and STROBE guidelines for the reporting of cohort studies [17].
Data source
All clinical information at Moorfields Eye Hospital is recorded within an electronic medical record (EMR) application (OpenEyes Foundation, London, UK). A SQL database (SQL Server Reporting Service, Microsoft Corporation, Richmond, USA) containing all the information from the EMR is in place and regular updates are performed overnight. VA is reported in ETDRS letter score. The highest value (independent of measurement method) available at each visit was chosen.
Participants
A data-warehouse query for patients that received one IVI for DMO (between March 2013 and October 2018) resulted in 3226 unique eyes from 2368 patients. Exclusion criteria were those that: (1) suffered from macular oedema secondary to other conditions than diabetes; (2) under 18 years old; (3) received fewer than three IVI; (4) received bevacizumab, dexamethasone intravitreal implant or fluocinolone acetonide intravitreal implant; leaving 2614 eyes of 1964 patients taken forward for analysis.
Treatment regimen
Patients that were included in this study received anti-VEGF therapy according to the recommended National Institute for Health and Care Excellence (NICE) guidelines at the injection clinic of Moorfields Eye Hospital NHS Foundation Trust, which is approved by the Clinical Audit and Effectiveness Committee (Fig. 1) [18].
Study outcomes
The primary study outcome is time-to-event analyses (Kaplan–Meier plot) for absolute VA attaining 70 ETDRS letters or above and Cox proportional-hazards modelling to identify predictive covariates. VA was recorded as the best value of the days visit (without correction, glasses or pinhole). Secondary event outcomes include time to VA <70 (≤ 69) and time to VA loss ≥ 15. Time-to-event analyses were employed to obviate the survival bias encountered in traditional visual outcome at given time point metrics (e.g., mean change in VA at year 2), which typically discounts absent patient data. Moreover, by incorporating all the available data leading up to missing values in our models for VA outcomes, they ought to reflect real life more accurately.
To enable comparison with previously published functional outcomes, we also carried out: (1) mean VA and change in VA per study eye as compared to baseline in ETDRS letters; (2) proportion of eyes with change in VA being <10 ETDRS letters and ≥10 ETDRS letters; and (3) proportion of eyes with ≥15 ETDRS letters gain or loss. For these analyses, selected observation time points and their definitions were as follows: baseline (date of first IVI); 1 year (12 months; 365 ± 90 days); 2 years (24 months; 720 ± 150 days); 3 years (36 months; 1095 ± 150 days); 4 years (48 months; 1460 ± 150 days).
Statistical methods
For statistical analyses, all EMR data were handled in R [19]. Time to each of the visual outcomes was visualised with Kaplan–Meier time-event plots. Cox proportional-hazards regression models were also carried out to evaluate the effects of demography (gender, ethnicity), clinical features at baseline (age and VA) and IVI (included as time-dependent covariates). Distribution of data was tested by the Shapiro–Wilk normality test. Means of non-parametric groups were compared using Wilcoxon signed-rank, Wilcoxon rank-sum and Kruskal–Wallis tests as appropriate. For more than two groups, multiple pairwise analyses was carried out with the Wilcoxon rank-sum test. Calculated means in text and figures are expressed with ± error margin corresponding to the standard deviation, unless otherwise specified. A p value < 0.05 was considered statistically significant.
Data sharing agreement
An anonymised version of the data set as well as the code used for analysis is available in the open-source digital repository Dryad—https://doi.org/10.5061/dryad.pzgmsbcfw. Depersonalisation was carried out through hash function anonymisation of patient identification numbers, and replacement of appointment dates with follow-up days to baseline. Approval of adequate depersonalisation was obtained by Moorfields Information Governance.
Results
Patient characteristics
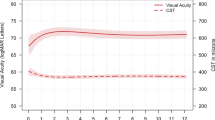
Our cohort comprised 2614 eyes of 1964 patients who initiated and completed a loading course of anti-VEGF therapy within the 4-year observation period (Supplementary Fig. 1). We compiled a data set that includes demographic information (Table 1) and all available VA values and IVIs from baseline (initiating of therapy) through to end of observation period. At baseline, the mean VA was 61.0 ± 15.3 ETDRS letters. For each of the annual time points, we saw an increasing number of eyes with missing VA values. This was largely due to the treatment duration for a given patient being shorter than the time point itself, leaving few due to loss-to-follow-up (LTFU). For instance, of the 1995 eyes without data at the 4-year time point, only 2 were lost-to-follow-up with the remainder having a treatment duration < 4 years. In total, 1147 eyes received aflibercept only, 707 eyes received ranibizumab only and 762 eyes received a combination (switch) between the agents.
Mean visual acuity trends are similar to existing literature
Trends in VA were compared to previously published studies. Mean VA changes and IVIs were comparable to retrospective cohort studies at 1- and 2-year time points (Supplementary Table 1). Data for comparison of 3- and 4-year time points do not yet exist. A change in VA of 10 and 15 letters is frequently considered when evaluating outcomes in patients with DMO receiving anti-VEGF therapy [13, 15, 20]. Also in keeping with reported retrospective studies, the proportion of eyes in this cohort gaining ≥10 letters was >30% and exceeded those who lost ≥10 letters at each of the annual time points (Supplementary Fig. 2a) [13, 15, 20]. A similar trend was observed when considering the proportion of eyes which gained ≥15 letters, with 19.3, 20.5, 18.9 and 21.3% of eyes observed at the annual time points spanning 1–4 years following baseline (Supplementary Fig. 2b).
Predictive factors for positive visual outcomes
Absolute VA ≥ 70 ETDRS letters is commonly used to measure positive visual outcomes in a clinical setting. Kaplan–Meier modelling of our cohort data suggests that 50% of DMO eyes are likely to reach VA ≥ 70 at 1.9 months after starting anti-VEGF therapy and over 75% after a year (Fig. 2a). To identify predictive variables for VA ≥ 70 in our cohort, we used Cox proportional-hazards modelling to relate VA outcomes to clinical time-dependent (anti-VEGF injections) and time-independent (gender, age at baseline, VA at baseline) covariates. This suggests that number of IVI, being male and—to a greater extent—VA at baseline are positively associated with VA ≥ 70 (Table 2). Indeed, the time point at which 50% of people are likely to reach VA ≥ 70 is much sooner in those with higher baseline VA of 60–69 (2.3 months) than with a lower baseline VA of 36–49 (57.0 months) (Fig. 2b). In contrast, age at baseline correlates inversely with VA ≥ 70 (Table 2) and with a smaller impact on event probability. A small, but statistically significant, difference in median event time is even seen between patients at either extreme of age—0.9 months in those ≤44 years of age compared with 5.1 months in those ≥75 (Fig. 2c).
Time from starting anti-VEGF injections to visual acuity (VA) reaching 70 ETDRS (Early Treatment Diabetic Retinopathy Study) letters or more was modelled (a). Cohorts were further stratified by statistically significant predictors (Table 2): baseline visual acuity (b) and age at first injection (c).
Interrogating duration to vision loss
The majority of our cohort attained the positive clinical outcome of VA ≥ 70 during the 48-month observation period (1995 of 2614 eyes). We subsequently interrogated the duration for which the VA remained at or above 70 in this sub-cohort by modelling time between attaining VA ≥ 70 to falling below 70 (≤69 letters). Of patients that reach VA ≥ 70, 50% are likely to fall below 70 at 14.7 months (Fig. 3a). Statistically significant predictive variables for falling below 70 after having attained VA ≥ 70 are VA at baseline (Fig. 3b), age at baseline (Fig. 3c) and injection number (Table 2). Interestingly, gender does not contribute significantly to VA falling below 70 as it does for reaching 70.
All patients attaining visual acuity (VA) ≥ 70 ETDRS (Early Treatment Diabetic Retinopathy Study) letters in the observation period were taken forward for analysis. Date of reaching this threshold was considering baseline, with time to VA falling below 70 modelled (a). Cohorts were further stratified by statistically significant predictors (Table 2): baseline visual acuity (b) and age at first injection (c). Of note, the sub-cohort of patients that began with VA at or over 70 letters (red line; b) was included in sub-stratified by baseline VA and featured median event time almost twofold greater than the other sub-cohorts.
Predictive variables for negative visual outcomes
A significant loss in vision has often been defined as loss of 15 ETDRS letters in the research of macular diseases as a synonym for three lines decline on Snellen chart. It is also recommended by the FDA as a relevant outcome for studies addressing macular diseases [21]. We observed a trend of male sex reducing the risk of losing more than 15 ETDRS letters during therapy. Despite the strong effect of hazard ratio (HR) of 0.88 this predictive factor was not statistically significant. The risk of losing more than 15 letters increases the longer eyes are observed (Fig. 4a). Independent predictors for losing more than 15 letters were a low VA at baseline (Fig. 4b) as well as a low number of injections (Table 2). As expected, age at baseline did not contribute as a predictor for unfavourable vision loss [22].
Date of injection 1 was considered baseline and time to visual acuity (VA) of 15 ETDRS letters or more was modelled (a). Cohort was further stratified by the statistically significant predictor (Table 2) baseline VA (b).
Discussion
Main findings
Here we report on the largest retrospective cohort of patients with DMO patients (2614 eyes of 1964 patients) receiving anti-VEGF therapy and over the longest observation period (4 years) to date. Reaching VA of 70 ETDRS letters or greater is an indicator of patient independence. Indeed, it is: (1) used as the mark for low vision alongside visual field loss and loss of contrast sensitivity [23, 24]; (2) the threshold for driving [25]; (3) the minimum VA required to read small print [26]; and therefore chosen as key positive VA outcome. The majority of our cohort achieved VA ≥ 70 (n = 1995; 76%) over the observation period and was likely to do so shortly after initiating anti-VEGF therapy (median survival at 1.9 months; Fig. 2). However, it is unknown for how long a given eye can expect to remain above this critical level. Our analyses demonstrate that the median survival of this sub-cohort to remain at or above 70 is 14.7 months (Fig. 3). That is, the VA in 50% of eyes will fall below 70 ETDRS letters 14.7 months after reaching ≥70. Baseline VA, baseline age and IVI number are predictive covariates for VA reaching and remaining above 70 (Table 2). An interpretation of this data is that early recognition of DMO indicated for treatment can increase the probability of positive visual outcomes; including VA reaching 70 ETDRS letters and extending the duration one remains above it [4, 5, 27].
Our results in context to randomised clinical trials
Demography and trends in VA of our cohort are consistent to those in similar real-world data reports in DMO (Supplementary Table 1) [8, 12,13,14,15, 20, 28, 29]. However, the annual mean VA changes observed in our cohort were less than those reported in the RCTs that led to approval of the anti-VEGF treatment (Table 1) [3,4,5, 30]. It has been postulated that these differences in IVI regimen and number account for this. For instance, the mean IVIs delivered over 24 months in the RISE (20.9) and RIDE (21.9) studies [4] are twofold greater than in any study reporting real-world data, including ours (8.9; Table 1). Our cohort analyses support this as it demonstrates that the number of IVIs as a positive predictive covariate of VA reaching VA ≥ 70 (HR: 1.18 [95% CI: 1.14–1.22]) and protective for negative VA outcomes—VA falling below 70 (HR: 0.96 [95% CI: 0.93–0.99]) and VA loss ≥ 15 (HR: 0.98 [95% CI: 0.96–1.00]) (Table 2). It is important to note that our model represents the Moorfields treatment protocol and incorporates IVIs as a time-dependent covariate. As such, patients that receive IVIs could signify greater disease severity. It is notable that IVIs are statistically significant predictors of positive visual outcomes in spite of this.
Another potential reason for the discrepancy in annual mean VA change between RCTs and our study is a difference in baseline VA. Our cohort features a greater mean baseline VA than the RCTs (61.0 vs. 56.9 letters in the RISE/RIDE study). It is established that baseline VA is inversely correlated with mean VA changes at 1 and 2 years in patients with DMO receiving anti-VEGF therapy [31]. Our model identifies baseline VA as a protective factor for positive outcomes: VA reaching 70 or more ETDRS letters (HR = 1.09 [95% CI: 1.09–1.1]) and remaining above 70 (HR = 0.97; [0.96–0.97]). That is, although those with a low baseline VA may exhibit a greater VA increase at 1 and 2 years, they are less likely to reach a positive visual outcome than those with a higher baseline VA.
Our results in context to real-world data
Several retrospective cohort studies have investigated 1-year visual outcomes in patients with DMO treated by anti-VEGF according to a PRN treatment regimen [8, 11,12,13, 15, 20] (Supplementary Table 1). In line with our findings, these real-world data studies reported comparable gains in VA from baseline after 1 year of treatment. Our data identify better baseline VA, higher number of IVIs and male gender as independent positive predictors for a favourable visual outcome (reaching >70 letters). Baseline VA has been described in meta-analysis as an independent predictive factor for VA outcomes in anti-VEGF treatment for DMO [32]. This is why real-life results with a lower baseline VA (48 letters) report higher gains in VA (+10.7 letters) as compared in this study by only delivering 5.4 injections [20]. By identifying a higher number of IVIs as positive time-dependent predictor, results from large meta-analysis can be confirmed. Injections number seems to be the number one reason for superior VA outcomes in RCTs compared to real life [31]. Interestingly, besides baseline VA and number of injections, male gender results in superior VA outcomes during therapy. Recently published real-life data found worse VA in female eyes compared to male eyes despite the same number of injections after 1 year of therapy [33]. This is supported by our data and subgroup analysis to compare gender-related outcomes should be performed on a larger scale.
With 3103 eyes at baseline, the United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group presented data from 19 attending centres on 1- and 2-year VA outcomes for the largest cohort of eyes so far with a gain of 3.1 and 1.4 letters, respectively [14]. Notably, these VA changes are lower than those observed in our study, which may reflect the multicentre setting (as opposed to our single centre study) and a considerable LTFU. Although Egan et al.’s data feature a high number of eyes at baseline, there is marked LTFU at subsequent time points—~60% at year 1 and 90% at year 2.
At present, there are little real-life data available that describe outcomes beyond 2-years following initiation of treatment. A retrospective study from Freiburg, Germany reported on VA outcomes for 479 eyes with DOM treated by a PRN regime over 5 years [11]. They observed between 12.7 and 16% of eyes gaining more than 15 letters in year 1–4 and delivered 6,2,2 and 1 anti-VEGF injection per study eye within 4 years of treatment. Considering the higher amount of injections administered (8,4,4 and 4), we report that 19–21% of eyes are gaining more than 15 letters during therapy.
Addressing missing data
LTFU is a general and challenging limitation in retrospective cohort data when evaluating outcomes at given time points. This is also the case in studies looking at anti-VEGF in DMO. LTFU rates reported in literature are as high as 13%, 31%, 48% and 65% after 1, 2, 3 and 4 years, respectively; and even up to 95% after 2 years in multicentre analysis [8, 14]. Our data featured comparable missing data at key time points, i.e., 27%, 41%, 60% and 76% at 1, 2, 3, and 4 years, respectively (Table 1). We therefore scrutinised the underlying reason for missing data by considering the treatment duration for each eye. As expected, we saw that the majority of missing data (>85% at each time point) were due to treatment duration being shorter than the time point (Table 1). For example, a patient with DMO initiating treatment in 2018 will not have a value any of the annual time points and it would be inappropriate to label them as LTFU. Through this consideration alone, our data set features the lowest LTFU rate reported amongst similar studies.
We also considered whether values were missing due to patients being deceased and is the first of such studies to do so. Interestingly, data values from 163 eyes of 102 patients were absent due to death over a 4-year observation period which extrapolates to 30.6 deaths per 1000 person years. This is higher than what has been previously reported in DM (26 deaths per 1000 person years) [34]. This correlation may be explained over the number of comorbidities besides diabetes that are present in the UK population [35]. Moreover, one would expect our study cohort (patients receiving hospital treatment for complications of DM) to be at higher risk of comorbidities than the general DM population.
Limitations of our study
Our data set attempts to account for missing data and consequently features the lowest LTFU rate amongst similar studies. Although this is the case, it is important to note that missing values remain, and these would be ignored in traditional time point analyses e.g. mean change in VA at year 2. Here it would be controversial to assume that patients with absent data at a given time point (death or otherwise) are a random selection of those that initiate treatment. As such, survival bias would feature prominently if mean values at given time points were generalised to all patients with DMO that undergo anti-VEGF therapy. Accordingly, we have employed time-to-event analyses as they use all data up until the point that someone has an event or no longer followed up. Moreover, time-independent and -dependent covariates were incorporated to enable adjustment for age, baseline VA, gender and injections. To calculate event probability per unit time, two key assumptions are taken. First, those who are censored have the same event probability as those who continue to be followed. This is difficult to test for as censoring can occur for various reasons. Second, time of study enrolment does not affect event probabilities.
In patients with bilateral DMO involvement and receiving IVIs, both eyes were included in our analyses. This is a possible confounder as it could lead to multiple testing. However, this could be addressed by other workgroups using our open-source data set.
Future implications
To date, this is the largest single centre cohort study reporting on real-life outcomes of anti-VEGF in DMO. There are several similar real-world data reports in DMO (Supplementary Table 1). Of these, our cohort size is larger than most of them combined. Comparable cohort size has been reported by Egan et al. [14]; a UK-wide multicentre study with 19 attending centres and thus various distinct treatment regimes, as opposed to a single protocol in our single centre study. Our inclusion criteria are broader than in the pivotal RCTs, including patients with higher and lower baseline VA, thus granting a more realistic account of DMO treatment outcomes.
By identifying a higher number of injections as clinical time-dependent variable for positive VA outcomes over 4 years (reaching VA > 70 letters), results of this study highlight the importance of treatment adherence and patient’s guidance to maintain a sufficient high treatment frequency. Especially in the light of patients with DMO showing a moderately low treatment adherence to regular injections compared to other indications (e.g., age-related macular degeneration) [16]. A research focussed key output of this study is to make an anonymised version of our data set available on an open-source data repository as a resource for all clinical researchers globally. This is with the aim of optimising the capacity of our data to positively impact clinical research and outcomes. By including data beyond the 2-year time horizon, we hope to expand current insight into long-term visual outcomes by enabling comparison and meta-analysis with future data that also report beyond 2 years. There is an increasing call for research that is transparent and that can have its key findings reproduced by its audience [36, 37]. This is of particular concern in clinical research predicated on digitalisation of healthcare environments, as collective progress can be hampered by the absence of detailed methodology and data sharing. To further address this, we have published a step-by-step guide written in open-source code R of how we performed our statistical analyses. This code can be directly applied to the published database to replicate all figures and values in this study.
Conclusions
Analyses of our cohort reveal that the majority reach 70 ETDRS letters or more whilst receiving their initial loading dose. Of these, the median survival for remaining above 70 is 14.7 months. Furthermore, we demonstrate that age, baseline VA and injection number are independent predictors of visual outcomes—suggesting that earlier diagnosis and treatment of DMO could increase the likelihood of positive outcomes. Lastly, this is the largest retrospective cohort study of anti-VEGF in DMO over the longest observation period to date and we have made this data set available.
Summary
What was known before
-
Visual acuity outcomes in randomised clinical trials are superior to results achieved in real-life cohorts.
-
To what extend this is caused by patient demographics (broader inclusion criteria) or just the lower number of anti-VEGF injections delivered remains unclear.
-
Retrospective analysis struggles with a high loss-to-follow-up rate, causing uncertainty in interpreting results beyond the 2-year time horizon.
What this study adds
-
This is the largest real-life dataset of eyes treated with anti-VEGF for diabetic macular oedema, covering a 4-year time horizon with minimal loss-to-follow-up.
-
By publishing this anonymised data set further research under the aspect of “open-science” is promoted.
-
Using survival analysis stratifies the impact of gender, age, number of injections and visual acuity at baseline on visual acuity outcomes.
Change history
15 July 2020
This Article was updated shortly after publication as the authors only wanted colour for the HTML version of this paper (originally, the PDF had colour too).
References
Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50.
Sabanayagam C, Yip W, Ting DSW, Tan G, Wong TY. Ten emerging trends in the epidemiology of diabetic retinopathy. Ophthalmic Epidemiol. 2016;23:209–22.
Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117:1078–86.e2.
Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801.
Korobelnik J-F, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–54.
Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–405.
Granström T, Forsman H, Olinder AL, Gkretsis D, Eriksson JW, Granstam E, et al. Patient-reported outcomes and visual acuity after 12 months of anti-VEGF-treatment for sight-threatening diabetic macular edema in a real world setting. Diabetes Res Clin Pract. 2016;121:157–65.
Maggio E, Sartore M, Attanasio M, Maraone G, Guerriero M, Polito A, et al. Anti-VEGF treatment for diabetic macular edema in a real-world clinical setting. Am J Ophthalmol. 2018. https://doi.org/10.1016/j.ajo.2018.08.004.
Kiss S, Liu Y, Brown J, Holekamp NM, Almony A, Campbell J, et al. Clinical utilization of anti-vascular endothelial growth-factor agents and patient monitoring in retinal vein occlusion and diabetic macular edema. Clin Ophthalmol. 2014;8:1611–21.
Fong DS, Luong TQ, Contreras R, Jimenez JJ, Custis PH, Patel V, et al. Treatment patterns and 2-year vision outcomes with bevacizumab in diabetic macular edema. Retina. 2018;38:1830–8.
Wecker T, Ehlken C, Bühler A, Lange C, Agostini H, Böhringer D, et al. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol. 2017;101:353–9.
Holekamp NM, Campbell J, Almony A, Ingraham H, Marks S, Chandwani H, et al. Vision outcomes following anti-vascular endothelial growth factor treatment of diabetic macular edema in clinical practice. Am J Ophthalmol. 2018;191:83–91.
Patrao NV, Antao S, Egan C, Omar A, Hamilton R, Hykin PG, et al. Real-world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom National Health Service Setting. Am J Ophthalmol. 2016;172:51–7.
Egan C, Zhu H, Lee A, Sim D, Mitry D, Bailey C, et al. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group, Report 1: baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. Br J Ophthalmol. 2017;101:75–80.
Best A-L, Fajnkuchen F, Nghiem-Buffet S, Grenet T, Quentel G, Delahaye-Mazza C, et al. Treatment efficacy and compliance in patients with diabetic macular edema treated with ranibizumab in a real-life setting. J Ophthalmol. 2018;2018:1–7.
Weiss M, Sim DA, Herold T, Schumann RG, Liegl R, Kern C, et al. Compliance and adherence of patients with diabetic macular edema to intravitreal anti-vascular endothelial growth factor therapy in daily practice. Retina. 2018;38:2293–300.
Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–24.
Gibson J. A review of the NICE guidance for the treatment of diabetic macular oedema. Br J Healthc Manag. 2015;21:502–4.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. 2018. http://www.R-project.org/.
Hrarat L, Fajnkuchen F, Boubaya M, Lévy V, Sarda V, Grenet T, et al. Outcomes after a 1-year treatment with ranibizumab for diabetic macular edema in a clinical setting. Ophthalmologica. 2016;236:207–14.
Center for Drug Evaluation and Research, U.S. Food and Drug Administration. Lucentis (ranibizumab) injection. Company: Genentech Inc. Application no.: 125156. Approval date: 06/30/2006. Rockville (MD): FDA; 2006. [cited 2019, Aug 21]. Medical review [Internet] (FDA drug approval package). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/125156s0000_Lucentis_MedR.pdf. Accessed 21 Aug 2019.
Mathur R, Bhaskaran K, Edwards E, Lee H, Chaturvedi N, Smeeth L, et al. Population trends in the 10-year incidence and prevalence of diabetic retinopathy in the UK: a cohort study in the Clinical Practice Research Datalink 2004–2014. BMJ Open. 2017;7:e014444.
Vision Rehabilitation Preferred Practice Pattern. https://www.aao.org/Assets/5872cf6f-104c-4a7a-a9f7-7a2bf506112a/636492820096070000/vision-rehabilitation-final-12-19-17-pdf. Accessed 11 Aug 2019.
Visual Standard Report International Council of Ophthalmology. http://www.icoph.org/downloads/visualstandardsreport.pdf. Accessed 14 Aug 2019.
Bron AM, Viswanathan AC, Thelen U, de Natale R, Ferreras A, Gundgaard J, et al. International vision requirements for driver licensing and disability pensions: using a milestone approach in characterization of progressive eye disease. Clin Ophthalmol. 2010;4:1361.
Lamoureux EL, Hassell JB, Keeffe JE. The impact of diabetic retinopathy on participation in daily living. Arch Ophthalmol. 2004;122:84–8.
Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open. 2014;4:e004015.
Lukic M, Williams G, Shalchi Z, Sim D, Patel PJ, Keane PA, et al. Intravitreal aflibercept for diabetic macular oedema: Moorfields’ real-world 12-month visual acuity and anatomical outcomes. Eur J Ophthalmol. 2019;30:1120672119833270.
The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203.
Prünte C, Fajnkuchen F, Mahmood S, Ricci F, Hatz K, Studnička J, et al. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 2015;100:787–95.
Ziemssen F, Feltgen N, Holz FG, Guthoff R, Ringwald A, Bertelmann T, et al. Demographics of patients receiving Intravitreal anti-VEGF treatment in real-world practice: healthcare research data versus randomized controlled trials. BMC Ophthalmol. 2017;17:7.
Virgili G, Parravano M, Evans JR, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2018;10:CD007419.
Schiefelbein J, Müller M, Kern C, Herold T, Liegl R, Fasler K, et al. Gender-related differences in patients treated with intravitreal anti-vascular endothelial growth factor medication for diabetic macular oedema. Eur J Ophthalmol. 2020:1120672119899627.
Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41.
Chudasama YV, Khunti KK, Zaccardi F, Rowlands AV, Yates T, Gillies CL, et al. Physical activity, multimorbidity, and life expectancy: a UK Biobank longitudinal study. BMC Med. 2019;17:108.
Stupple A, Singerman D, Celi LA. The reproducibility crisis in the age of digital medicine. npj Digital Med. 2019;2. https://doi.org/10.1038/s41746-019-0079-z.
Celi LA, Citi L, Ghassemi M, Pollard TJ. The PLOS ONE collection on machine learning in health and biomedicine: towards open code and open data. PLoS ONE. 2019;14:e0210232.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mixed competing interests: “All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: KB reports grants from Bayer AG, personal fees from Alimera, from Allergan, outside the submitted work; PAK reports other from Big Picture Eye Health, during the conduct of the study; personal fees from DeepMind, personal fees from Optos, personal fees from Novartis, personal fees from Bayer, personal fees from Allergan, personal fees from Heidelberg Engineering, personal fees from Topcon, personal fees from Carl Zeiss Meditec, personal fees from Haag Streit, personal fees from Santen, grants from the National Institute for Health Research, grants from Fight For Sight UK, outside the submitted work; KK reports personal fees from Big Picture Eye Health, during the conduct of the study, grants and personal fees from Novartis Pharma, grants and personal fees from Bayer Pharma, personal fees from Zeiss, personal fees from Allergan, personal fees from Alcon, personal fees from Google Deepmind; DAS reports other from Big Picture Eye Health, during the conduct of the study; personal fees from Allergan, personal fees from Novartis, personal fees from Bayer, personal fees from Big Picture Eye Health, personal fees from Haag Streit, outside the submitted work; PJP reports grants and other from Bayer, other from Novartis, outside the submitted work; no other relationships or activities that could appear to have influenced the submitted work.” RH, CK, DJF, JH, SKW and LF have nothing to disclose. The authors declare no conflicts of interests that could appear to have influenced the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kern, C., Fu, D.J., Huemer, J. et al. An open-source data set of anti-VEGF therapy in diabetic macular oedema patients over 4 years and their visual acuity outcomes. Eye 35, 1354–1364 (2021). https://doi.org/10.1038/s41433-020-1048-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-1048-0
This article is cited by
-
Cost-Effectiveness of Faricimab in the Treatment of Diabetic Macular Oedema (DMO): A UK Analysis
PharmacoEconomics - Open (2024)