Abstract
Objective
To evaluate the risk of stroke associated with intravitreal ranibizumab in age-related macular degeneration (AMD).
Methods
A nationwide retrospective case-crossover study was performed using data from the Korean National Health Insurance Service (KNHIS) database, which included patients with exudative AMD in South Korea (n = 41,860). The index date was the date of hospitalization for stroke. We defined the case period as 60 days and four control periods before the index date. A pharmacy prescription database was searched for ranibizumab use during the case and control periods. We calculated adjusted odds ratios (ORs) and 95% confidence intervals (CIs) with a conditional logistic regression model.
Results
A total of 865 patients with AMD and incident stroke were included. Of all the patients, 12.02% had been treated during the preceding 60-day case period, compared with 9.25–10.29% during control periods. The adjusted OR of stroke associated with intravitreal ranibizumab during the case period was 1.285 (95% CI 0.979–1.686) (p = 0.07). In the subgroup analysis, the risk of hemorrhagic stroke had an OR of 2.252 (95% CI 1.068–4.749, p = 0.033). Further analyses based on patient gender, age, and different risk periods of 15 and 30 days yielded no increase in the risk of stroke associated with intravitreal ranibizumab.
Conclusions
This case-crossover analysis revealed no evidence of increased risk of hospitalization for stroke within 60 days of intravitreal ranibizumab injection in AMD patients. A secondary analysis indicated the possibility of an increased risk of hemorrhagic stroke, with borderline significance. Further research is needed regarding the underlying biological mechanisms and drug safety.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is a leading cause of blindness worldwide [1]. After establishment of their efficacy to treat exudative AMD, vascular endothelial growth factor (VEGF) inhibitors have been the mainstay for treatment [1, 2]. VEGF has a pivotal role in the homeostasis of blood vessels outside of the retina, including those in the heart and brain. VEGF stimulates the production of nitric oxide, which has multiple vasculoprotective effects, including vasodilation, antithrombotic activity, and angiogenesis [3]. Inhibition of VEGF has an antiangiogenic action, is associated with thrombogenicity, and thus might increase the risk of cardiovascular events, including stroke.
Ranibizumab has been the most widely used intravitreal VEGF inhibitor and is also the most intensively evaluated drug for its efficacy and safety through numerous randomized trials. The ANCHOR clinical trial showed a higher rate of arterio-thromboembolic events in those receiving 0.5-mg doses of ranibizumab than in those receiving a 0.3-mg dose or verteporfin injection, but the incidence of adverse events was too low to show statistical differences [2]. The MARINA trial showed that the rate of arterio-thromboembolic events was 4.6% in patients treated with 0.3 or 0.5 mg ranibizumab compared with a rate of 3.8% in those who received sham injections; none of the differences were significant [1]. The meta-analysis of the data from the MARINA, ANCHOR, and FOCUS trials revealed an increased but nonsignificant risk of cerebrovascular accident in patients receiving monthly ranibizumab injections compared with the corresponding risk in patients receiving sham injections [4]. A large observational cohort study using administrative claims data included 147,000 subjects and found no difference in stroke risk with ranibizumab compared with the stroke risk from photodynamic therapy (PDT), pegaptanib, or bevacizumab [5]. On the other hand, some studies have shown a correlation between ranibizumab and stroke or thromboembolic events. A recent meta-analysis involving 11 trials comprising 6596 patients with AMD showed a significant increase in systemic vascular adverse events with more intensive treatment [6]. In the SAILOR study, nonvascular death, stroke, and hemorrhage rates were higher in the 0.5 mg ranibizumab group than in the 0.3 mg group [7]. Despite the unquestionable effectiveness of VEGF inhibitors in restoring and improving the vision of patients with exudative AMD, the possible adverse effects on the systemic vasculature remain uncertain.
In this study, we performed a case-crossover study to investigate the association of intravitreal ranibizumab use with the risk of stroke in patients with exudative AMD using nationwide medical claims data in South Korea.
Methods
Statement of ethics
This retrospective nationwide cohort study design was reviewed and approved by the Institutional Review Board of the National Health Insurance Service Ilsan Hospital, Gyeonggi-do, Korea. (NHIMC 2015-02-021) The study adhered to the tenets of the Declaration of Helsinki, and written informed consent was waived.
Case-crossover study
We used a case-crossover study design to assess the relationship between intravitreal ranibizumab injections and risk of stroke in exudative AMD patients. Instead of using matched control subjects, the past experience of the case served as the case’s own control. Therefore, time-invariant confounders, including those that could not be measured, are controlled for by design. The case-crossover design has been widely used as a tool to evaluate drug safety and is particularly suitable when the exposure is intermittent, the effect on risk is immediate and transient, and the outcome is abrupt. The available data suggested that the potential anti-VEGF antibody-related systemic effect would be immediate and transient [8] and that the outcome (stroke) would be abrupt, such that the case-crossover design was an appropriate method in this study.
Database
This study used data from the NHIS-NCS 2002–2013 (NHIS-2015-1-070), which was released by the Korean National Health Insurance Service (KNHIS). The database included the entire population of Korea (n = 47,990,760), and we used the data from 2009 through 2014.
All Korean residents are obligated to enroll in the KNHIS. Claims are accompanied by data regarding sociodemographic characteristics, diagnostic codes, hospitalization, procedures, prescription drugs, and hospital characteristics. No patient healthcare records are duplicated because all Korean residents receive a unique identification number at birth. The KNHIS uses the Korean Classification of Diseases (KCD), which is a system similar to the International Classification of Diseases. In 2007, the KNHIS initiated a copayment reduction of up to 90% for patients suffering from 138 rare and intractable disorders, including exudative AMD. Patients with exudative AMD who registered in the program were eligible for the copayment reduction after receiving a confirmed diagnosis by an ophthalmologist based on the KNHIS diagnostic criteria. After registration, all exudative AMD-related claims contain the exudative AMD registration code (V201) in addition to the diagnostic code for exudative AMD (H3531).
Study sample
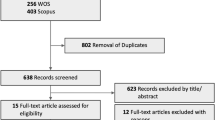
We identified 41,860 patients with newly diagnosed exudative AMD (mean age 69.89 ± 8.97) using the registration code for exudative AMD (V201) among beneficiaries 40 years of age and older during the 6-year study period (2009–2014). Among these patients, 3809 patients were hospitalized with a primary diagnosis for acute cerebrovascular events (coded as KCD I60, I61, and I62 for hemorrhagic stroke and I63 for ischemic stroke). Because the data were available from 2005, we established incident exudative AMD diagnosis by applying a 4-year washout period to remove any potential preexisting exudative AMD (n = 1261). In addition, patients who were hospitalized with stroke before the registration for exudative AMD were excluded (n = 1683), leaving a final sample of 865 patients with an incident stroke after their exudative AMD diagnosis (mean age 75.58 ± 7.49). The onset of stroke (index date) was defined as the date of acute stroke hospitalization. Figure 1 presents the flowchart of subject enrollment.
Data on index drug exposure and potential confounding factors
Ranibizumab (0.5 mg only) has been reimbursed by the NHIS since 2009. We collected prescription drug and intravitreal injection data on the use of ranibizumab. We included patients with both prescription (electronic data interchange code 653600320/E01631541) and intravitreal injection (S5070) codes on the same day; thus, there was no time lag between the drug prescription and intravitreal injection. We collected information on the patients’ age, gender, number of injections, and comorbidities, including myocardial infarction, diabetes mellitus, hypertension, congestive heart failure, dementia, chronic lung disease, peptic ulcer disease, chronic liver disease, and hemiplegia based on KCD codes (see Supplementary Table 1, which lists KCD codes for patients’ comorbidities). These factors were selected as candidate risk factors due to their potential associations with the risk of stroke. These risk factors also served as potential effect modifiers for assessing the association between intravitreal ranibizumab injection and risk of stroke.
Statistical analysis
For each patient, we defined the case period as 1–60 days and four control periods as 121–180, 181–240, 241–300, and 301–361 days before the index date. Pharmacy prescription and intravitreal injection databases were searched for ranibizumab use during the case and control periods. We compared intravitreal ranibizumab injection between case and control periods and calculated crude odds ratios (ORs) and their 95% confidence intervals (CIs) by conditional logistic regression. A significance level of 0.05 was selected. The SAS statistical package for Windows, version 9.4 (SAS Institute, Inc., Cary, North Carolina, USA) and Stata/SE version 12.1 (StataCorp, College Station, TX, USA) was used to perform the analyses in this study.
Results
Characteristics of the study subjects
Table 1 lists characteristics of the study cohort of 865 patients with acute stroke and exudative AMD. More than half (52.95%) of the patients were in their 70s, and 60% of the patients were men. The type of stroke was mainly ischemic (85.66%), and the other strokes (14.34%) were hemorrhagic. Patients had various systemic comorbidities, including hypertension (80.92%), followed by peptic ulcer disease (67.51%), chronic pulmonary disease (67.17%), and diabetes (57.92%). When the household income of the patients was analyzed, the majority of patients were in the group over 70 percentile. A total of 24.05% of the patients lived in a large city, and the rest of the patients’ residences were distributed in the order of metropolitan (16.30%), small city (16.01%), and rural areas (13.64%).
Intravitreal ranibizumab injections
The total number of intravitreal ranibizumab injections was 540, including multiple injections per patient. Of the 865 patients, 276 had received at least one injection within 1 year before the incident stroke, 254 (29.36%) had received 1–3 injections, and 22 (2.54%) had received more than 4 injections. A total of 589 (68.09%) patients had no injection history within this period (Table 1). Of all the patients, 12.02% had been treated during the preceding 2-month case period, compared with 9.25–10.87% during control periods (Table 2).
Effect of intravitreal ranibizumab injections on the risk of incident stroke
In the multivariate logistic regression analyses, the crude OR and covariate-adjusted OR of stroke associated with intravitreal ranibizumab during the preceding 2-month case period were 1.296 (95%CI 0.995–1.688) (p = 0.05) and 1.285 (95% CI 0.979–1.686) (p = 0.0705), respectively, which were not statistically significant. In the subgroup analysis, intravitreal ranibizumab injection was significantly associated with an elevated risk of hospitalization for hemorrhagic stroke (OR: 2.252, 95% CI 1.068–4.749, p = 0.033). For gender and age, intravitreal ranibizumab injection did not increase the risk of incident stroke within the 60-day case period (Table 3). Furthermore, in addition to the defined case and control periods, we conducted an analysis using different time windows (1–15 and 1–30 days for the case periods). There was no increase in the specific risk of stroke associated with intravitreal ranibizumab in different case periods (Table 4).
Discussion
Using national health insurance claim data, this case-crossover analysis found that intravitreal ranibizumab was not significantly associated with an increase in the risk of hospitalization for stroke within 60 days in wet AMD patients. Subgroup analyses based on age, gender, and different case periods also showed no increase in the risk of stroke associated with intravitreal ranibizumab. In the secondary analysis according to stroke subtype, increased risk of hospitalization for hemorrhagic stroke was observed with marginal significance.
Previous studies performed in AMD patients have demonstrated a controversy concerning the possible association between intravitreal anti-VEGF injection and stroke. Some studies have shown a statistically significant association between stroke and anti-VEGF injection [6, 7, 9], but others have not demonstrated a definite increased risk of stroke [2, 4, 10,11,12,13,14,15]. The case-series study with self-controlled design by Pratt et al. suggested an elevated risk of hospitalization due to ischemic stroke in patients on ranibizumab therapy for a 30–60-day risk period [9]. In the SAILOR study, nonvascular death, stroke, and hemorrhage rates were higher in the 0.5 mg ranibizumab group than in the 0.3 mg group [7]. A recent meta-analysis by Ueta et al. also showed a significant increase with 0.5 versus 0.3/0.0 mg for cerebrovascular accident (CVA), monthly versus pro re nata/0.0 mg for CVA and 0.3/0.5 versus 0.0 mg for nonocular hemorrhage [6].
However, there have been several studies showing contradictory results with a nonsignificant increase in systemic cardiovascular risk. The pooled analysis of data from several randomized clinical trials (RCTs) revealed that the rates of cerebrovascular events had an increasing tendency but no statistically meaningful difference for patients treated with ranibizumab versus sham: OR, 3.24; 95% CI, 0.96–10.95 [4], OR, 2.2; 95% CI, 0.8–7.1 [10]. A recent meta-analysis using RCTs demonstrated that the risk of cerebrovascular events was not associated with ranibizumab exposure with the hazard ratios, 1.25, 95% CI, 0.61–2.55 [11]. However, these RCTs used for meta-analysis were powered to evaluate the efficacy of ranibizumab treatment but not specifically to detect small differences in the rates of infrequent adverse events. In another meta-analysis, the effect of ranibizumab versus control treatment was not interpretable due to the small number of events (cerebrovascular events: n = 2, 2, 5 for the control, 0.3 mg ranibizumab, and 0.5 mg ranibizumab treatments, respectively) [12]. In the ANCHOR study, the overall rates of arterial thromboembolic events, including vascular death, myocardial infarction, and ischemic or hemorrhagic stroke, were slightly higher in the ranibizumab-treated group than in the PDT group, but the absolute number of adverse events was very low (three patients (2.2%) in the 0.3-mg group, six patients (4.3%) in the 0.5-mg group, and three patients (2.1%) in the verteporfin group) [2]. A population-based time series analysis reported that the use of bevacizumab and ranibizumab for AMD was not associated with a change in the rate of hospitalization for stroke among Ontario seniors with retinal disease [13]. The nested case-control study showed that exposure to ranibizumab within 180 days was not associated with significant risks of ischemic stroke [14]. A study using Korean national claims data demonstrated that ranibizumab treatment for AMD did not increase the overall risk of stroke compared with that in comorbidity-matched controls or sociodemographically matched controls [15]. These results are consistent with our findings that the risk of hospitalization for ischemic stroke, which is the major type of stroke, was not associated with intravitreal ranibizumab.
Few studies have analyzed the association of ranibizumab use with the incidence of stroke by subtypes such as ischemic and hemorrhagic. Most studies have been performed on stroke without specification of the subtype or have analyzed ischemic stroke only, and hemorrhagic stroke has not been treated separately. An analysis of the Medicare claims database indicated a 57% higher risk of hemorrhagic stroke with bevacizumab, with no statistically significant differences in the risk of either myocardial infarction or ischemic stroke (Gower EW, IOVS, 2011, 310, ARVO E-Abstract, 6644). To our knowledge, our study is the first to reveal a statistically significant association between the risk of hemorrhagic stroke and ranibizumab exposure. However, the mechanism underlying this association could not be directly elucidated from this result. One possible hypothesis is that anti-VEGF therapy-induced hypertension may increase intracranial hemorrhage. Hypertension is one of the most frequently reported side effects of systemic VEGF inhibition, which involves decreased production of nitric oxide in the wall of arterioles and other resistance vessels [16]. Another potential explanation is that AMD itself that is active enough to require ranibizumab treatment, rather than the direct effect of ranibizumab exposure, may be a risk factor for hemorrhagic stroke. Previous studies have reported that late or neovascular AMD is strongly associated with intracerebral hemorrhage but not with cerebral infarction [17, 18]. Further clinical studies are warranted to confirm our results and investigate the mechanisms.
Our study has several strengths. We applied a case-crossover design in the study of AMD patients to investigate the association between intravitreal ranibizumab injection and the risk of stroke, both ischemic and hemorrhagic. This design can effectively control for time-invariant confounders, such as smoking, body mass index, cholesterol level, and other chronic comorbidities (hypertension, diabetes, carotid artery disease, etc.), as each patient served as his or her own control. In addition, use of nationwide claims data offered generalizability and statistical power to evaluate incident ischemic and hemorrhagic stroke in AMD patients, which is relatively rare.
There are some limitations to our study. First, we could not control the off-label use of intravitreal bevacizumab injection because it is not covered by the KNHIS. It is possible that off-label use of bevacizumab would have attenuated the association of ranibizumab and stroke. Second, although we used a self-controlled study design to reduce confounding, the change in systemic medications or newly developed cerebrovascular risk factors during the study period (i.e., time-varying confounding) might have affected the risk of stroke. The observed differences in the risk of hemorrhagic stroke might have been affected by possible confounders. In this study, we adjusted for hospitalizations for cardiovascular diseases, dementia, DM, and hypertension, but did not adjust for changes in systemic medications. Third, because we used large-scale claim data from KNHIS, the sensitivities of the diagnosis codes for determining the risk factors of stroke had not been validated by a direct review of the medical records. However, previous studies have reported that the accuracy of the diagnoses in the KNHIS data is quite reliable [19, 20]. Fourth, as this study was based on the interpretation of insurance claim data, we could not distinguish between AMD and variant AMD, such as polypoidal choroidal vasculopathy and retinal angiomatous proliferation, which share the same diagnostic code. Since there is a difference in the pathophysiology of these diseases, there is a possibility that the incidence of stroke after ranibizumab use may also have differed. Another limitation is that most patients were exposed to <3 ranibizumab injections during a year before the index date, which is a lower exposure than that in average clinical settings. This might have diminished the impact of the ranibizumab injections on risk of stroke. Last, this study excluded patients with a stroke history prior to the diagnosis of AMD to identify incident stroke cases. However, considering that patients with a history of stroke might have a higher risk with use of anti-VEGF therapy, the risk of stroke as related to ranibizumab injection might have been underestimated by the exclusion of these patients. Therefore, further studies are needed to evaluate the risk in patients with a history of stroke.
In conclusion, this case-crossover analysis revealed no evidence of increased risk of hospitalization for ischemic stroke within 60 days of intravitreal injection of ranibizumab in AMD patients. A secondary analysis indicated the possibility of an increased risk of hemorrhagic stroke. Further research is needed regarding the underlying biological mechanisms and drug safety.
Summary
What was known before
-
Ranibizumab has been the most widely used intravitreal VEGF inhibitor and is also the most intensively evaluated drug for its efficacy and safety through numerous randomized trials.
-
Despite the unquestionable effectiveness of VEGF inhibitors in restoring and improving the vision of patients with exudative AMD, the possible adverse effects on the systemic vasculature remain uncertain.
What this study adds
-
This case-crossover analysis found no evidence of an increased risk of hospitalization for stroke within 60 days of intravitreal ranibizumab administration in AMD patients.
-
A secondary analysis indicated the possibility of an increased risk of hemorrhagic stroke with marginal significance. Further research is needed regarding the underlying biological mechanisms and drug safety.
References
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N. Engl J Med. 2006;355:1419–31.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44.
Tunon J, Ruiz-Moreno JM, Martin-Ventura JL, Blanco-Colio LM, Lorenzo O, Egido J. Cardiovascular risk and antiangiogenic therapy for age-related macular degeneration. Surv Ophthalmol. 2009;54:339–48.
Ueta T, Yanagi Y, Tamaki Y, Yamaguchi T. Cerebrovascular accidents in ranibizumab. Ophthalmology. 2009;116:362.
Curtis LH, Hammill BG, Schulman KA, Cousins SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010;128:1273–9.
Ueta T, Noda Y, Toyama T, Yamaguchi T, Amano S. Systemic vascular safety of ranibizumab for age-related macular degeneration: systematic review and meta-analysis of randomized trials. Ophthalmology. 2014;121:2193–203 e1–7.
Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116:1731–9.
Xu L, Lu T, Tuomi L, Jumbe N, Lu J, Eppler S, et al. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci. 2013;54:1616–24.
Pratt NL, Ramsay EN, Kemp A, Kalisch-Ellett LM, Shakib S, Caughey GE, et al. Ranibizumab and risk of hospitalisation for ischaemic stroke and myocardial infarction in patients with age-related macular degeneration: a self-controlled case-series analysis. Drug Saf. 2014;37:1021–7.
Bressler NM, Boyer DS, Williams DF, Butler S, Francom SF, Brown B, et al. Cerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trials. Retina. 2012;32:1821–8.
Zarbin MA, Francom S, Grzeschik S, Tuomi L, Haskova Z, Macfadden W, et al. Systemic safety in ranibizumab-treated patients with neovascular age-related macular degeneration: a patient-level pooled analysis. Ophthalmol Retin. 2018;2:1087–96.
Dugel PU, Singh N, Francom S, Cantrell RA, Grzeschik SM, Fung AE. The systemic safety of ranibizumab in patients 85 years and older with neovascular age-related macular degeneration. Ophthalmol Retin. 2018;2:667–75.
Campbell RJ, Bell CM, Paterson JM, Bronskill SE, Moineddin R, Whitehead M, et al. Stroke rates after introduction of vascular endothelial growth factor inhibitors for macular degeneration: a time series analysis. Ophthalmology. 2012;119:1604–8.
Campbell RJ, Gill SS, Bronskill SE, Paterson JM, Whitehead M, Bell CM. Adverse events with intravitreal injection of vascular endothelial growth factor inhibitors: nested case-control study. BMJ. 2012;345:e4203.
Rim TH, Lee CS, Lee SC, Kim DW, Kim SS. Intravitreal ranibizumab therapy for neovascular age-related macular degeneration and the risk of stroke: a national sample cohort study. Retina. 2016;36:2166–74.
Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–95.
Lee WA, Cheng CL, Lee CH, Kao Yang YH, Lin SJ, Hsieh CY. Risks of newly onset hemorrhagic stroke in patients with neovascular age-related macular degeneration. Pharmacoepidemiol Drug Saf. 2017;26:1277–85.
Wieberdink RG, Ho L, Ikram MK, Koudstaal PJ, Hofman A, de Jong PT, et al. Age-related macular degeneration and the risk of stroke: the Rotterdam study. Stroke. 2011;42:2138–42.
Kim H, Yun JE, Lee SH, Jang Y, Jee SH. Validity of the diagnosis of acute myocardial infarction in Korean national medical health insurance claims data: the Korean heart study (1). Korean Circ J. 2012;42:10–5.
Park JK, Kim KS, Kim CB, Lee TY, Lee KS, Lee DH, et al. The accuracy of ICD codes for cerebrovascular diseases in medical insurance claims. Korean J Prev Med. 2000;33:76–82.
Acknowledgements
This work was supported by a National Health Insurance Ilsan Hospital grant (NHIMC 2015-02-015). This study used data from the NHIS-NCS 2002–2013 (NHIS-2015-1-070), which was released by the KNHIS. The authors alone are responsible for the content and writing of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, J., Kim, D.W., Kim, D.H. et al. Risk of stroke associated with intravitreal ranibizumab injections in age-related macular degeneration: a nationwide case-crossover study. Eye 35, 601–607 (2021). https://doi.org/10.1038/s41433-020-0911-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0911-3
This article is cited by
-
Risk analysis for patients with arterial thromboembolic events after intravitreal ranibizumab or aflibercept injections
Scientific Reports (2023)
-
Risk of Myocardial Infarction, Stroke, or Death in New Users of Intravitreal Aflibercept Versus Ranibizumab: A Nationwide Cohort Study
Ophthalmology and Therapy (2022)
-
Effect Modification by Indication to the Risks of Major Thromboembolic Adverse Events in Patients Receiving Intravitreal Anti-Vascular Endothelial Growth Factor Treatment: A Population-Based Retrospective Cohort Study
BioDrugs (2022)
-
Comparative Risk of Arterial Thromboembolic Events Between Aflibercept and Ranibizumab in Patients with Maculopathy: A Population-Based Retrospective Cohort Study
BioDrugs (2021)