Abstract
Purpose
To improve upon self-reported glaucoma status in population-based cohorts by developing a questionnaire-based proxy incorporating self-reported status in conjunction with glaucoma-specific visual complaints.
Methods
A vision specific questionnaire, including questions from the National Eye Institute Visual Functioning Questionnaire-25 (NEI-VFQ-25) was administered to 79,866 Lifelines participants, a population-based cohort study in the Northern Netherlands. We compared NEI-VFQ-25 responses between ‘definite’ glaucoma cases (n = 90; self-reported surgical cases) and an age- and gender-matched subset of controls (n = 1,800) to uncover glaucoma-specific visual complaints, using a case–control logistic regression. We defined ‘probable glaucoma’ as both self-reported disease status and visual complaints, and ‘possible glaucoma’ as either. To evaluate the resulting proxy, we determined age-stratified glaucoma prevalences in the remaining cohort and compared the result to the literature.
Results
Per unit increase in the vision subscales (range 0–100) distance, peripheral and low luminance, we observed significantly increased odds of definite glaucoma (2% [P = 0.03], 4% [P = 1.2 × 10−8] and 2% [P = 0.02], respectively); the associated area under the curve was 0.73. We identified 300 probable and 3,015 (1,434 by self-report) possible glaucoma cases. Standardised prevalences of definite, probable and possible glaucoma for 55+ were 0.4%, 1.1% and 7.3%, respectively. For self-reported glaucoma (combining definite, probable and possible by self-report), this was 5.2%.
Conclusions
The combination of self-reported glaucoma status and visual complaints can be used to capture glaucoma cases in population-based settings. The resulting prevalence of combined definite and probable glaucoma (1.5%) appears to be more consistent with previous reports than the prevalence estimate of 5.2% based only on self-report.
Similar content being viewed by others
Introduction
Open-angle glaucoma (OAG) is an adult-onset progressive eye disease that can eventually lead to irreversible blindness, and is the second leading cause of permanent visual impairment among the elderly worldwide [1]. Hallmarks of glaucomatous damage are excavation of the optic nerve head, thinning of the retinal nerve fibre layer, and loss of retinal ganglion cells [2]. These structural changes can be observed with fundoscopy, fundus photography, as well as optical coherence tomography, and lead to defects in the visual field that can be identified via perimetry [3]. An epidemiological definition of glaucoma was developed in 2002 by the International Society for Geographical and Epidemiological Ophthalmology (ISGEO), and is still routinely used [4]. Briefly, glaucoma is classified in three categories, primarily based on the presence of structural and functional abnormalities, with IOP readings and medical history used as additional information. Phenotyping all participants for glaucoma in epidemiological studies according to the ISGEO definition requires costly and time-consuming ophthalmic assessments. While doable—and highly valuable—in studies with 1,000–10,000 participants [5,6,7,8,9], current enrolment sizes can exceed 100,000 participants [10, 11], driving the search for innovative, data-driven solutions.
One solution for phenotyping participants in large datasets is the use of questionnaire data. Currently in the UK-biobank, a population-based cohort, glaucoma is classified via self-reported status [12]. While successful for associating glaucoma with socioeconomic status [13], relying on a self-reported diagnosis has limitations. First, half of the glaucoma patients are unaware of their disease status [14,15,16]. Second, and possibly more important, there is ocular hypertension, a condition with a much higher prevalence than glaucoma and often mistaken as glaucoma by the patient. Ocular hypertension is a risk factor for glaucoma, but the majority of these patients will never develop glaucoma, i.e. there are no obvious structural changes and their visual function remains intact. Hence, the question is, is it possible to make a better questionnaire-based proxy for glaucoma than self-reported disease status only?
Although glaucoma is often considered asymptomatic until end-stage disease, this is actually not the case. Many patients, including those with early-stage glaucoma, report problems in extreme luminance settings, see Bierings et al. [17] for recent data and discussion on this topic. These reported subjective visual complaints suggest that a questionnaire-based proxy for glaucoma is feasible. The subjective visual experience of the glaucoma patient can be evaluated with existing visual function questionnaires and a well-known example is the National Eye Institute 25-Item Visual Function Questionnaire (NEI-VFQ-25). The NEI-VFQ-25 was developed and validated in a number of populations, and in different ocular disorders [18], indicating disease specific response patterns [19, 20].
The aims of this study were (1) to improve upon self-reported glaucoma status in population-based cohorts by developing a questionnaire-based glaucoma proxy that incorporates self-reported status in conjunction with glaucoma-specific subjective visual experience, and (2) to evaluate the feasibility of our glaucoma proxy in the Lifelines cohort, a large (n ~ 167,000) population-based study conducted in the Netherlands. For this purpose, we compared answers to the NEI-VFQ-25 questions between separately identified ‘definite’ glaucoma cases and an age- and gender-matched subset of controls, with a multivariable case–control logistic regression. Using the resulting coefficients from the regression to construct a classification equation, together with information on self-reported glaucoma or high eye pressure, we defined ‘probable’ and ‘possible’ glaucoma. As a feasibility check, we used the resulting proxy (classification into definite, probable, and possible glaucoma based on questionnaire data) to describe the age-specific prevalence of glaucoma in Lifelines.
Methods
Lifelines participants
Lifelines is a prospective population-based cohort study of the Northern Netherlands [21]. It examines the health and health-related behaviours of 167,729 persons, in a unique three-generation design. For this, Lifelines employs a broad range of investigative procedures in assessing the biomedical, socio-demographic, behavioural, physical and psychological factors which contribute to the health and disease of the general population, with a special focus on multi-morbidity and complex genetics [22]. The cohort structure, where participants will be followed for at least 30 years, is described fully elsewhere [11]. The NEI-VFQ-25 questionnaire was administered to all participants aged 18 and older during the first follow-up visit between 2014 and 2017, and the first wave of results (n = 79,866 adult participants) was used for this study. This sample is similar to the remaining adult portion of the Lifelines cohort measured at baseline, 59% female vs 58% in the rest of the cohort, and 50.4 years of age compared with 50.8 in the remaining population. Those who self-identified as Caucasian were included in the analyses.
The study protocol was approved by the ethics board of the University Medical Center Groningen and adhered to the tenets of the Declaration of Helsinki. All participants in Lifelines provided written informed consent.
Questionnaire data
We used Part 2 of the NEI-VFQ-25 (‘difficulty with activities’ questions) in combination with self-reported eye disease status and current or past treatment for proxy development. Table 1 presents the questions used in the current study. The NEI-VFQ-25 driving questions were omitted due to excessive multicollinearity based on a variance inflation factor cut-off of 5, and missingness that exceeded 5% [23].
We used the scoring algorithm developed by Mangione et al. to transform initial visual question responses to visual subscales [24, 25]. Initial responses were ranked from 1 to 6, where a higher score within 1–5 indicates a worse visual problem. Response 6 indicates the person stopped the activity due to other reasons, and was treated as missing. Responses were transformed to a scale from 0–100; the higher the score, the poorer the visual experience. Imputation of missingness was performed for missing data up to 5% [24]. Question 9 in the NEI-VFQ-25 ‘distance vision’ subscale (‘Because of your eyesight, how much difficulty do you have going down steps, stairs, or curbs in dim light or at night?’) was used to create a low luminance subscale, as glaucoma patients struggle in low luminance situations [17, 24]. The resulting six visual subscales were peripheral (Question 6 in Table 1), distance vision (Questions 4 and 10), near vision (Questions 1–3), social functioning vision (Question 7 and 9), colour vision (Question 8) and low luminance vision (Question 5). We aimed for primary glaucoma, where glaucoma is not secondary to another eye disease. Based on the available information regarding other eye diseases, and the likelihood that these diseases could result in secondary glaucoma, we excluded those who underwent surgery or laser intervention for diabetic retinopathy (Questions 14 and 18 in Table 1) or surgical intervention for retinal detachment (Question 17). Those diagnosed with age-related macular degeneration (AMD) were also excluded (Question 11). Although the visual complaint pattern of AMD will differ from that of glaucoma patients, it is a common age-related eye disease that might confound our case detection.
From questionnaire data to glaucoma proxy
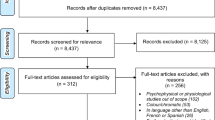
The development of the glaucoma proxy consisted of three steps. First, as mentioned above, we excluded those with a history of diabetic retinopathy, macular degeneration, or retinal detachment, aiming for primary glaucoma. Second, we defined and identified ‘definite glaucoma’ in the remaining participants, see Fig. 1. Third, we sampled an age- and gender-matched subgroup of controls with no self-reported eye disease, see Table 2. We compared NEI-VFQ-25 answers between the definite glaucoma cases and matched controls, together termed the ‘training population’. From this comparison, and from the answers to the additional questions displayed in Table 1, we defined ‘probable’ and ‘possible’ glaucoma. These steps together yielded a proxy; a classification into definite, probable and possible glaucoma, as well as unaffected, based on questionnaire data. What now follows are the details of the approach.
The flowchart describes the exclusion process, the training population and regression, as well as the classification process in the testing population. Refer to Table 1 for relevant questions.
Training population; definite glaucoma
Definite glaucoma was defined as a history of an incisional surgical intervention for glaucoma (Question 16 in Table 1). Subsequently, a group (n = 1,800) of 20 random controls per definite glaucoma case was created through propensity scoring of age and gender, using R (matchit with method = ‘nearest’, exact = ‘gender’, and ratio = 20) [26]. This population of definite cases and age- and gender-matched controls is now referred to as the ‘training population’, see Fig. 1.
Next, a case–control logistic regression of the visual subscales was performed in the training population, see Fig. 1. The resulting coefficients were used to build a classification equation for glaucoma class discrimination. A receiver operator characteristic (ROC) curve was constructed to identify thresholds for this class discrimination. Two classification thresholds were obtained. ‘Threshold 1’ was the maximum value of the Youden index, i.e. the largest distance between the ROC-curve and the chance line. ‘Threshold 2’ was more stringent, corresponding to 97.5% specificity on the ROC-curve.
Testing population; probable and possible glaucoma
The classification equation was then applied to the remaining Lifelines participants, the ‘testing population’ (entire cohort excluding those with conflicting eye diseases, the training population controls, and those who did not self-identify as Caucasian). Individual regression scores were tested against the thresholds described above. Participants with glaucoma-related treatments/diagnosis (Questions 12, 13 and 19 in Table 1) and surpassing Threshold 1 were classified as ‘probable’. Participants with glaucoma-related treatments/diagnosis but not surpassing Threshold 1 were classified ‘possible by self-report’. Participants surpassing Threshold 2 without glaucoma treatment/diagnosis were considered ‘possible by complaint’. The two classes of ‘possible’ were then combined. The remaining participants were classified as ‘unaffected’ (see Fig. 1).
Performance of glaucoma proxy
To determine the feasibility of this proxy in a population-based setting, the prevalence of glaucoma was obtained. Age-stratified (up to 55, 55–69, 70+) glaucoma prevalences were obtained through the number of definite, probable, and possible cases divided by the total number of participants in the concerning age-stratum. Prevalences were then standardised via the Dutch 2018 population census (obtained from Statistics Netherlands, https://opendata.cbs.nl/statline/#/CBS/en/dataset/37296eng/table?fromstatweb, accessed December 6th, 2018), and compared with the literature.
Data analysis
All analyses were performed using SPSS (version 24.0.0.2) and R (version 3.4.2). A P value of 0.05 or less was considered statistically significant in all analyses.
Results
Of the 79,866 submitted questionnaires, 79,845 participants provided usable data. The remaining 21 participants did not complete the eye portion of the questionnaire. Ages ranged from 19 to 94, with a mean age (standard deviation) of 50.4 (12.6) years. In total, 2,339 participants were removed due to either conflicting eye diseases, or for identifying other than Caucasian, resulting in 77,506 participants (Fig. 1).
In total there were 111 participants who had a history of glaucoma surgery. After excluding those who underwent laser treatment or surgery for diabetes (n = 2), and/or surgery for retinal detachment (n = 10; one overlapping with diabetic laser/surgery treatment), and/or who had AMD (n = 13; three overlapping with retinal detachment surgery), 90 cases of definite glaucoma were identified within the Lifelines population. The mean (standard deviation) age of the definite glaucoma cases was 61.7 (12.6) years. The age of the definite glaucoma cases was significantly different than the rest of the cohort (paired t-test P = 6.4 × 10−15); after selecting 20 age- and gender- matched controls (n = 1,800), age was not significantly different between cases and controls (paired t-test P = 0.89). Table 2 displays the mean visual subscale scores of the definite glaucoma cases and the age- and gender-matched controls. In the univariable analyses, all subscales were highly significantly different between cases and controls. In the multivariable analysis, per one unit increase in the vision subscales (range 0–100) of distance, peripheral, and low-luminance vision, we observed significantly increased odds of definite glaucoma of 2% (P = 0.03), 4% (P = 1.2 × 10−8), and 2% (P = 0.02), respectively. Figure 2 shows the ROC curve for the logistic regression. The area under the curve (AUC) was 0.73. The corresponding classification equation was:
Threshold 1 was 0.08 with an associated sensitivity of 50.0% and specificity of 89.8%. Threshold 2 was 0.17 with an associated sensitivity of 30.0% at the required specificity of 97.5%.
In total, 300 participants were classified as probable glaucoma and 3,015 as possible glaucoma. Of these 3,015 possible glaucoma cases, 1,434 participants were classified as possible by self-report and 1,581 as possible by complaint. The remaining 72,301 participants were classified as ‘unaffected’ (Fig. 1).
Prevalence
Table 3 presents the prevalence of glaucoma, stratified by age and glaucoma category. Age-stratified prevalences ranged from 0.06% of definite cases in the youngest age stratum up to ~9% of possible cases in the oldest age stratum. The raw prevalences of definite, probable, and possible glaucoma in the 55+ population were 0.2%, 0.9%, and 6.6%, with corresponding standardised prevalences of 0.4%, 1.1%, and 7.3%, respectively. For self-reported glaucoma (combining definite, probable, and possible by self-report), the standardised prevalence was 5.2%.
Discussion
We created a questionnaire-based glaucoma proxy for use in population-based epidemiology; the resulting glaucoma categories are definite, probable, possible, and unaffected. Categorisation is based on a history of glaucoma surgery, self-reported glaucoma and ocular hypertension which includes self-reported medical treatment for glaucoma/ocular hypertension, as well as the presence or absence of glaucoma-specific visual complaints according to the NEI-VFQ-25. Based on this proxy, the standardised prevalence of combined definite and probable glaucoma in the 55+ population participating in Lifelines is ~1.5%.
Our definitions of definite, probable, and possible glaucoma differ, inevitably, from the ISGEO criteria, and do not correspond one-to-one to clinical diagnoses. For the ISGEO Category 1 glaucoma diagnosis (the strictest category), glaucomatous visual field loss (GVFL) and glaucomatous optic neuropathy (GON) are required. In clinical settings, patients who have undergone glaucoma surgery likely fulfil the ISGEO Category 1 diagnosis criteria; many medically treated patients fulfil these criteria as well. Hence, our definite cases are a subset of the ISGEO Category 1 diagnosis cases. As mentioned in the Introduction, it is not possible to simply rely on self-reported disease status to define glaucoma in population-based epidemiology, as the prevalence of ocular hypertension is much higher than that of glaucoma, and both are easily mixed-up by patients [27]. To address this, we combined self-reported disease status/treatment with glaucoma-specific visual complaints; this combination was denoted as probable glaucoma. In the Rotterdam Study, the prevalence of glaucoma was 0.8% in the 55+ population when using ISGEO Category 1 diagnosis (GVFL and GON) [15]. If we extend the glaucoma classification to what was used more recently in the Rotterdam Study [28, 29], from ISGEO Category 1, to GVFL with or without GON together with ISGEO Category 2 diagnosis (GON only, but with a stricter criterion), the prevalence of glaucoma in the Rotterdam Study is 3.2%. As all participants receive a full eye exam, the Rotterdam Study identifies both patients who are aware of their disease status, as well as those with undiagnosed glaucoma [30]. As mentioned in the Introduction, only half of the glaucoma patients are aware of their disease status, thus the prevalence of 3.2% in the Rotterdam Study corresponds quite well with our 1.5%, given that our definitions of definite and probable glaucoma imply awareness. As Lifelines and the Rotterdam Study are both based in the Netherlands and comprise Caucasian participants only, our proxy provides a prevalence estimate that is consistent with diagnosed glaucoma if we combine definite and probable glaucoma.
In the UK Biobank, the age-stratified prevalence of self-reported glaucoma was 0.48% for participants 40–49 years of age, 1.18% for those 50–59 years of age, and 2.68% for those aged 60–69 [13]. Considering that glaucoma is generally diagnosed in the sixth decade of life, the UK Biobank population is relatively young [31]. To compare these prevalences to our dataset, we calculated the corresponding prevalences for the combined definite and probable categories. This yielded a prevalence of 0.27% for participants 40–49 years of age, 0.37% for those 50–59 years of age, and 1.29% for those aged 60–69. Given that the prevalence of definite and probable categories combined appears to approximate that of diagnosed glaucoma in the general population quite well (see previous paragraph), the self-report approach of the UK biobank seems to yield an overestimation of the actual glaucoma prevalence when assuming the same glaucoma prevalence in the Caucasian populations of the UK and the Netherlands. As mentioned in the Introduction, spurious inclusion of ocular hypertension could explain this overestimation. To compare, the prevalence of self-reported glaucoma in our study (combined definite, probable, and possible by self-report categories) was 1.50% for participants 40–49 years of age, 2.40% for those 50–59 years of age, and 4.50% for those aged 60–69. These values seem higher than those reported by the UK biobank. This difference likely arose as we explicitly mentioned ocular hypertension as part of the self-reported glaucoma question (Question 12 in Table 1), where the UK biobank did not (The question for participants was, ‘Has a doctor told you that you have any of the following problems with your eyes’ with ‘glaucoma’ in a list of ocular disorders).
In Lifelines, participants not aware of their disease could have been captured in our study as ‘possible glaucoma based on complaint’. We obtained 1,581 possible by complaint, clearly exceeding the expected number (~400) based on a disease awareness of 50%. This category is presumably a mixture of glaucoma cases and false positives. Similarly, the possible glaucoma by self-report category is likely made up of participants with early, asymptomatic glaucoma detected by chance, as well as glaucoma suspects, i.e. those with ocular hypertension, or, for example, those followed-up because of relatives with glaucoma. Depending on the purpose of future studies using our proxy, the possible glaucoma category could be excluded rather than being attributed to either cases or controls.
To evaluate the robustness of our approach to uncover the glaucoma-specific visual complaints, we re-analysed our data utilising linear discriminant analysis (LDA). The same NEI-VFQ-25 subscales were identified as relevant to glaucoma and no statistical difference was identified between the AUC of logistic regression, and that of LDA (AUC for logistic regression = 0.73, AUC for LDA = 0.73, P = 0.76) [32]. The LDA categorisation was as follows, 366 for probable (290 overlapping with logistic regression), 1,368 for possible by self-report (1,358 overlapping with logistic regression), and 1,907 possible by complaint (1,563 overlapping with logistic regression). The LDA was less conservative in classifying ‘probable’ and ‘possible glaucoma by complaint’, however, identified cases largely overlapped between the two approaches. The large overlap of glaucoma categorisation for both approaches suggests robustness of our approach.
We aim to study primary OAG. Potentially, our data are contaminated with primary angle-closure glaucoma, pseudoexfoliative glaucoma, secondary glaucoma, or other ocular disorders that may present as glaucoma through the subjective visual complaints. Fortunately, primary angle-closure glaucoma and pseudoexfoliative glaucoma have a significantly lower prevalence in our Caucasian study population [30]. A systematic review reported the prevalence ratio of primary OAG compared with primary angle-closure glaucoma in European-derived populations to be ~10:1 (range: 2.6:1 to 20.7:1) [33]. Similarly, at the population level, secondary glaucoma is rare compared with primary OAG [34], but this might be different in a clinical setting [35]. So, to further reduce the probability of spuriously including secondary glaucoma, we excluded those who underwent surgery or laser intervention for diabetic retinopathy, or surgical intervention for retinal detachment. Finally, retinal dystrophies like retinitis pigmentosa present with a similar subjective vision loss pattern to glaucoma, i.e. deficits in peripheral and low luminance vision, but are rare in the general population with an estimated prevalence below 0.1% in the Netherlands [36, 37].
A major strength of this study was the stringent inclusion criterion for definite glaucoma, being a history of glaucoma surgery, enabling us to quantify the glaucomatous visual impairment very clearly. A limitation is that history of glaucoma surgery was self-reported, and may have been reported erroneously. Due to the nature and privacy of the data, medical records were unavailable to us. As such, the subjective visual complaints obtained from this study may be moderated by misclassification, resulting in an underestimation of the power of a questionnaire-based glaucoma proxy. Specifically, mixing-up glaucoma surgery and cataract surgery may have occurred. To address this, cataract surgery was addressed separately in the questionnaire, before glaucoma surgery. The use of 20 age- and gender-matched controls per case allows us to surmise that the vision loss captured in the regression is due to glaucoma, and not general ageing. Our method was developed in a Caucasian population. It is unknown if the proxy can be used in populations of different ethnicities. However, it was reported that glaucoma patients of African descent have no difference when compared with Caucasian glaucoma patients with respect to the NEI-VFQ-25 visual subscales [38]. Although no direct comparison to a Caucasian population was made, Korean glaucoma patients also have reported significant issues with the peripheral and social function vision subscales [39], and in a case–control comparison of Latino-Americans, significant differences were observed in all visual subscales [40]. A limitation of this study is the exclusion of participants with diabetic retinopathy, retinal detachment, and AMD—intentionally done to avoid including participants with secondary glaucoma. As such, no conclusions can be made regarding glaucoma in patients with ophthalmic comorbidity.
In conclusion, this study demonstrated that glaucoma patients have significantly poorer subjective visual experiences than their age- and gender-matched peers in terms of distance, low luminance, and peripheral vision as assessed by questionnaires. Their subjective visual experience, together with self-reported disease status, successfully guides the creation of a classification equation to rank others’ glaucoma proxy status. The resulting prevalence appears to be more consistent with previous glaucoma prevalence literature than a prevalence estimate based only on self-report. The proxy opens opportunities for glaucoma research within large-scale population-based settings, where in-depth phenotyping of all participants is not easily feasible.
Summary
What was known before
-
Self-reported glaucoma in population-based cohorts may be inaccurate as up to half of people with glaucoma are unaware of their disease status.
What this study adds
-
Including glaucoma-specific vision loss in combination with self-reported disease status adds robustness to classifying glaucoma in population-based cohorts where participants did not receive eye exams.
Change history
30 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41433-021-01511-3
References
Quigley HA. Number of people with glaucoma worldwide. Am J Ophthalmol. 1996;122:460.
Janssen SF, Gorgels TGMF, Ramdas WD, Klaver CCW, van Duijn CM, Jansonius NM, et al. The vast complexity of primary open angle glaucoma: disease genes, risks, molecular mechanisms and pathobiology. Prog Retin Eye Res. 2013;37:31–67.
Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–64.
Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42.
Wild PS, Zeller T, Beutel M, Blettner M, Dugi KA, Lackner KJ, et al. The Gutenberg Health Study. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55:824–9.
Hofman A, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, Ikram MA, et al. The Rotterdam Study: 2014 objectives and design update. Eur J Epidemiol. 2013;28:889–926.
Tielsch JM, Katz J, Singh K, Quigley HA, Gottsch JD, Javitt J, et al. A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol. 1991;134:1102–10.
Iwase A, Suzuki Y, Araie M, Yamamoto T, Abe H, Shirato S, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111:1641–8.
Wang YX, Xu L, Yang H, Jonas JB. Prevalence of glaucoma in North China: the Beijing Eye Study. Am J Ophthalmol. 2010;150:917–24.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779.
Scholtens S, Smidt N, Swertz MA, Bakker SJL, Dotinga A, Vonk JM, et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol. 2015;44:1172–80.
Chua SYL, Thomas D, Allen N, Lotery A, Desai P, Patel P, et al. Cohort profile: design and methods in the eye and vision consortium of UK Biobank. BMJ Open. 2019;9:e025077.
Shweikh Y, on behalf of the UK Biobank Eye and Vision Consortium, Ko F, Chan MPY, Patel PJ, Muthy Z, et al. Measures of socioeconomic status and self-reported glaucoma in the UK Biobank cohort. Eye. 2015;29:1360–7.
Hollows FC, Graham PA. Intra-ocular pressure, glaucoma, and glaucoma suspects in a defined population. Br J Ophthalmol. 1966;50:570–86.
Wolfs RC, Borger PH, Ramrattan RS, Klaver CC, Hulsman CA, Hofman A, et al. Changing views on open-angle glaucoma: definitions and prevalences–The Rotterdam Study. Invest Ophthalmol Vis Sci. 2000;41:3309–21.
Patty L, Wu C, Torres M, Azen S, Varma R. Validity of Self-reported Eye Disease and Treatment in a Population-based Study: The Los Angeles Latino Eye Study. Ophthalmology. 2012;119:1725–30.
Bierings RAJM, van Sonderen FLP, Jansonius NM. Visual complaints of patients with glaucoma and controls under optimal and extreme luminance conditions. Acta Ophthalmol. 2018;96:288–94.
Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric Properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). Arch Ophthal. 1998;116:1496–504.
Cheng H-C, Guo C-Y, Chen M-J, Ko Y-C, Huang N, Liu CJ-L. Patient-reported vision-related quality of life differences between superior and inferior hemifield visual field defects in primary open-angle glaucoma. JAMA Ophthalmol. 2015;133:269–75.
Abe RY, Diniz-Filho A, Costa VP, Gracitelli CPB, Baig S, Medeiros FA. The Impact of Location of Progressive Visual Field Loss on Longitudinal Changes in Quality of Life of Patients with Glaucoma. Ophthalmology. 2016;123:552–7.
Klijs B, Scholtens S, Mandemakers JJ, Snieder H, Stolk RP, Smidt N. Representativeness of the LifeLines Cohort Study. PLoS ONE. 2015;10:e0137203.
Stolk RP, Rosmalen JGM, Postma DS, de Boer RA, Navis G, Slaets JPJ, et al. Universal risk factors for multifactorial diseases: LifeLines: a three-generation population-based study. Eur J Epidemiol. 2008;23:67–74.
Hair, JF, Anderson, RE, Babin, BJ, Black, WC. Multivariate Data Analysis (7th ed). (Pearson International, 2009).
Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, & Hays RD National Eye Institute Visual Function Questionnaire-25. PsycTESTS Dataset (2001) https://doi.org/10.1037/t08622-000.
Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8.
Sekhon, JS. Multivariate and Propensity Score Matching Software with Automated Balance Optimization: The Matching Package for R. J. Stat. Softw. 2011;42:1–52.
Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. discussion 829–30
Czudowska MA, Ramdas WD, Wolfs RCW, Hofman A, De Jong PTVM, Vingerling JR, et al. Incidence of Glaucomatous Visual Field Loss: A Ten-Year Follow-up from the Rotterdam Study. Ophthalmology. 2010;117:1705–12.
Springelkamp H, Wolfs RC, Ramdas WD, Hofman A, Vingerling JR, Klaver CC, et al. Incidence of glaucomatous visual field loss after two decades of follow-up: the Rotterdam Study. Eur J Epidemiol. 2017;32:691–9.
Stoutenbeek R, de Voogd S, Wolfs RCW, Hofman A, de Jong PTVM, Jansonius NM. The additional yield of a periodic screening programme for open-angle glaucoma: a population-based comparison of incident glaucoma cases detected in regular ophthalmic care with cases detected during screening. Br J Ophthalmol. 2008;92:1222–6.
Chen PP. Blindness in patients with treated open-angle glaucoma. Ophthalmology. 2003;110:726–33.
DeLong ER, DeLong DM, Clarke-Pearson DL. comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837.
Day AC, Baio G, Gazzard G, Bunce C, Azuara-Blanco A, Munoz B, et al. The prevalence of primary angle closure glaucoma in European derived populations: a systematic review. Br J Ophthalmol. 2012;96:1162–7.
Skenduli-Bala E, de Voogd S, Wolfs RCW, van Leeuwen R, Ikram KM, Jonas JB, et al. Causes of Incident Visual Field Loss in a General Elderly Population. Arch Ophthal. 2005;123:233–8.
de Vries MM, Muskens RPH, de Lavalette VWR, Hooymans JMM, Jansonius NM. Glaucoma drainage device surgery after vitreoretinal surgery: incidence and risk factors. Acta Ophthalmol. 2015;94:135–9.
Buch H, Vinding T, La Cour M, Nielsen NV. The prevalence and causes of bilateral and unilateral blindness in an elderly urban Danish population. The Copenhagen City Eye Study. Acta Ophthalmologica Scandinavica. 2001;79:441–9.
van Huet RAC, Oomen CJ, Plomp AS, van Genderen MM, Klevering BJ, Schlingemann RO, et al. The RD5000 database: facilitating clinical, genetic, and therapeutic studies on inherited retinal diseases. Invest Ophthalmol Vis. Sci. 2014;55:7355–60.
Ringsdorf L, McGwin G, Owsley C. Visual Field Defects and Vision-specific Health-related Quality of Life in African Americans and Whites With Glaucoma. J Glaucoma. 2006;15:414–8.
Kim YS, Yi MY, Hong YJ, Park KH. The impact of visual symptoms on the quality of life of patients with early to moderate glaucoma. Int Ophthalmol. 2018;38:1531–9.
Broman AT, Munoz B, Rodriguez J, Sanchez R, Quigley HA, Klein R, et al. The impact of visual impairment and eye disease on vision-related quality of life in a Mexican-American population: proyecto VER. Invest Ophthalmol Vis Sci. 2002;43:3393–8.
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement EGRET No 661883. The funding organisation had no role in the design, conduct, analysis, or publication of this research. The Lifelines Biobank initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG the Netherlands), University of Groningen and the Northern Provinces of the Netherlands.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The Acknowledgements section was incorrect.
Rights and permissions
About this article
Cite this article
Neustaeter, A., Vehof, J., Snieder, H. et al. Glaucoma in large-scale population-based epidemiology: a questionnaire-based proxy. Eye 35, 508–516 (2021). https://doi.org/10.1038/s41433-020-0882-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0882-4