Abstract
Background
Ciclo plasty using high-intensity focused ultrasound (HIFU) technology acts through the selective coagulation of the ciliary body. Our aim was to evaluate the safety and efficacy profiles of 8-s probe HIFU cyclocoagulation using the EyeOP1 device.
Methods
Prospective pragmatic trial. Inclusion criteria: adult glaucoma patients with uncontrolled IOP despite optimised medical therapy, and/or intolerant to medical therapy required to achieve target IOP. Primary outcome: surgical success defined as IOP reduction from baseline >20% with final IOP ≤21 mmHg, without adding any IOP-lowering drugs, and without loss of light perception; or decreased use of IOP-lowering drugs with stable/decreased IOP, without loss of light perception. Secondary outcomes: mean IOP, intra and postoperative complications, best-corrected visual acuity (BCVA) and number of IOP-lowering drugs at each visit. Outcome data were collected preoperatively and at postoperative day 1, and months 1, 3, 6 and 12.
Results
Forty-nine eyes of forty-nine patients (28 male) with a mean age of 70 ± 14 years were enroled. Pre-operative IOP was 26.9 ± 7.4 mmHg under 2.8 ± 0.9 topical medications, decreasing to 17.8 ± 6.4 mmHg under 2.3 ± 1 drugs at 12 months (p < 0.01). One-year surgical success was achieved in 71.4% of patients (IOP-reduction criteria: 59.2%; decreased use of IOP-lowering drugs: 38.8%). Eight patients were ultimately submitted to other glaucoma surgical interventions. Five patients experienced serious adverse events (loss of light perception n = 5; hypotony n = 1).
Conclusions
This innovative non-invasive technology seems to be effective in decreasing IOP and/or the number of administered drops in patients with refractory glaucoma. It seems a valuable tool to delay or preclude the need for filtering procedures in the majority of the patients.
Similar content being viewed by others
Introduction
Glaucoma is a chronic progressive optic neuropathy with an estimated prevalence of 64.3 million people worldwide, and projections are to further increase to 76.0 million by 2020 [1]. While several risk factors have been identified for disease onset and progression, intraocular pressure (IOP) remains the only actively modifiable factor—and so, the basis for therapeutic management of disease [2,3,4]. IOP control is not always straightforward to obtain, and according to two nationwide studies on glaucoma treatment patterns, 25–28% of patients are on multiple topical drug regimens [5, 6]. These may be candidates for glaucoma filtering surgery.
The impact of glaucoma on patients’ quality of life (QoL) is thus potentially enormous, considering not only the visual disability but also the burden of medical and/or surgical treatment [7]. The Collaborative Initial Glaucoma Treatment Study compared the QoL impact of initial medical with surgical approaches and concluded that, on the short term, QoL was affected in the surgical arm. While this difference became non-significant on the long term, it details how filtering surgery can be a stressful period for patients [8]. In fact, ongoing innovative trials (Treatment of Advance Glaucoma Study [9]) are still trying to determine whether primary surgical intervention has an advantage over medical therapy in newly diagnosed advanced cases.
A new balance between efficacy and safety was recently obtained by novelty technologies affecting the aqueous humour production in non-invasive manners [10,11,12,13]. Among these, Gazzard et al. demonstrated the better cost-effectiveness profile of the selective laser trabeculoplasty when compared with conservative topical drug regimens, while reducing the need for surgical interventions in the long run [14]. It is reasonable to consider the same may hold true for other non-incisional IOP-lowering techniques.
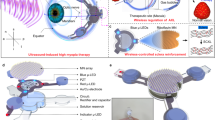
In this pragmatic trial we focus on the partial destruction of the ciliary body through high-intensity focused ultrasound (HIFU). This system performs selective cyclocoagulation trough a miniaturised circular system composed of six high frequency piezoelectric transducers, decreasing the aqueous humour production and therefore the IOP; a possible increase in the aqueous outflow through the uveoscleral route is another suggested effect mechanism [15, 16]. This technology enables a non-incisional, fast, and easy to learn procedure, with reports from previous 6-s probes demonstrating an average of 35% IOP reduction and a reported 65–70% response rate [15]. The purpose of this study was to evaluate the safety and efficacy profiles of HIFU cyclocoagulation using the new 8-s probe of EyeOP1 device (Eye Tech Care, Lyon, France) on a real life setting.
Materials and methods
We present the first-year results of a prospective pragmatic non-comparative trial ongoing at the Glaucoma department of a tertiary care centre (Hospital de Santa Maria, Lisboa, Portugal). This study was conducted in compliance with the Declaration of Helsinki. The Institutional Review Board approved the conduct of this study, and all patients provided both verbal and written informed consent before enrolment.
Up for inclusion were adult patients (i.e. ≥18 years old) diagnosed with open or closed-angle glaucoma, either primary or secondary, with uncontrolled IOP despite maximum tolerated medical therapy and/or intolerant to medical therapy required to achieve target IOP. Only one eye was allowed per study subject (first treated). Enrolment period was set between January 2015 and June 2017. Exclusion criteria included any visual field loss attributable to an unrelated condition. Lens status and previous incisional glaucoma surgery were not considered for purposes of patient selection.
Sample size was calculated as to detect a minimal difference of 20% in the rate of success, considering: alpha level of 0.05; power of 80%; null proportion of 0.1. We obtained an estimated sample size of 42 patients, adding an additional number of 5 patients to prevent for losses of information during follow-up.
All patients underwent a baseline multimodal assessment by the same investigator (LAP) for diagnosis and aetiology confirmation, and preoperative staging of disease. This evaluation included: best-corrected visual acuity (BCVA, decimal), slit lamp biomicroscopy, fundus examination, Goldmann applanation tonometry (three measurements), gonioscopy, automated visual field testing if possible (G Dynamic 30-2 test; Octopus, Haag-Streit, Koeniz, Switzerland) and peripapillary retinal nerve fibre layer scans using spectral domain optical coherence tomography (Heidelberg Engineering, Heidelberg, Germany).
All patients were treated according to the pre-specified surgical protocol, under combined regional anaesthesia and short sedation. HIFU cyclocoagulation was performed using the EyeOP1 device, which probe is equipped with six piezoelectric transducers. Three different probe diameters are available (11, 12 and 13 mm); selection was done according to preoperative biometric data, in order to better fit the ocular size. The probe cup was filled with balanced salt solution and manually centred on the patient’s eye. The treatment device was held in place by a suction system during the standardised sequential activation of the six sectors. We used standard (not customisable) HIFU treatment parameters, and were set as following: frequency 20.5 MHz; acoustic power 2.45 W; transducer activation time 8 s; time between shots 20 s.
Postoperative treatment included a topical fixed combination of tobramycin and dexamethasone given four times a day for 4 weeks. Considering the pragmatic study design, preoperative IOP-lowering drugs were initially maintained and further adjusted according to IOP values. Postoperative follow-up visits were scheduled at days 1 and 7, and months 1, 3, 6 and 12. At each visit, patients were submitted to a full ophthalmological observation (including three measurements of Goldmann applanation tonometry), and any experienced adverse events were recorded. Whenever patients were submitted to glaucoma invasive surgery (trabeculectomy or tube-shunt surgery) during the follow-up period, these cases were considered as failure, and censoring was performed to any data following the second intervention.
Primary composite outcome was the surgical success defined as final IOP ≤ 21 mmHg with >20% decrease in IOP from baseline, without adding any IOP-lowering drugs, and without loss of light perception; or decreased use of IOP-lowering drugs (topical and/or oral carbonic anhydrase inhibitor (CAI)), with stable/decreased IOP, without loss of light perception. Secondary outcomes: mean IOP, intra and postoperative complications, BCVA (Snellen decimal) and number of IOP-lowering drugs in use at each visit. For statistical purposes and according to Lange et al., “counting fingers” was classified as 0.01; “hand movements” as 0.005 and “light perception” as 0.0005 [17].
Statistical analysis was performed with STATA® v15 (StataCorp, Lakeway Drive, USA), at the significance level α of 0.05. Missing data were approached with a last observation carried forward strategy.
Results
Forty-nine eyes of forty-nine patients (28 male) were included in this analysis. Mean age was 70 ± 14 years old [range 24–88]. Mean preoperative IOP was 26.9 ± 7.4 mmHg, and patients were under an average of 2.8 ± 0.9 topical IOP-lowering drugs [range 0–4]. Seventeen patients were under oral CAI (35%). Mean central corneal thickness was 522.5 ± 39.2 µm. Mean baseline visual field severity, as assessed through mean deviation, was of 14.44 ± 8.12 [range 1.7–27.8]. Most eyes had primary open-angle glaucoma (49%), followed by secondary open-angle (29%), secondary angle closure (8%) and neovascular glaucoma (8%). Thirty-three eyes were pseudophakic (67%), and seven had previous glaucoma surgery (14.3%; single trabeculectomy n = 2, one of which with bleb revision; tube surgery n = 2; trabeculectomy + tube surgery n = 1; microinvasive glaucoma surgery n = 2, one of which with bleb revision). The proportion of patients naïve to filtering surgery was 89.8%. Mean follow-up time was 22.7 ± 5 months [range 12–30]. See Table 1 for baseline demographic data.
Primary outcome
One-year composite surgical success as previously defined was achieved in 35 eyes (71.4%). Among these, 29 eyes fulfilled the criteria of final IOP ≤ 21 mmHg with an IOP-reduction >20% without adding any IOP-lowering drugs and without loss of light perception (59.2%), and 19 had a decreased need for IOP-lowering drugs with stable/reduced IOP and without loss of light perception (38.8%).
Eight patients were submitted to glaucoma incisional surgical intervention before the 1-year time point; in these, the second surgical intervention was delayed by an average of 6.5 months [range 3–10].
Efficacy results are summarised in Table 2, and Table 3 details the cases of surgical failure.
Secondary outcomes
Mean preoperative IOP reduced from 26.9 ± 7.4 mmHg to: 16.5 ± 9.8 mmHg at day 1; 16.7 ± 7.4 mmHg at 1 month; 17 ± 7.4 mmHg at 3 months; 18.5 ± 7.9 mmHg at 6 months; and 17.8 ± 6.4 mmHg at 12 months post procedure (two-sided t-test p < 0.001 for the comparison of each time point to baseline). In average, at final observation, IOP was reduced from baseline by 34%.
The number of IOP lowering drugs was progressively reduced from 2.8 ± 0.9 at baseline, to 2.3 ± 1.0 at 1-year post procedure (two-sided t-test p < 0.001). Mean and relative IOP reductions from baseline are given in Fig. 1.
At month 3, all patients but one had discontinued oral CAI (percentage users: baseline—35%; month 3–2%; chi-square p < 0.001). At last observation, four patients were under oral CAI (8%; test of proportions p < 0.001), all of which were submitted to glaucoma filtering surgery before the 12-month endpoint—and so, considered as cases of treatment failure (Table 3).
At baseline, mean BCVA was 0.43 ± 0.32 (range 0.0005–1). No statistically significant differences were found in paired t-test regarding BCVA throughout the study period, except at the 12-month follow-up (1 month: 0.41 ± 0.3, p = 0.51; 3 months: 0.44 ± 0.28, p = 0.65; 6 months: 0.47 ± 0.32, p = 0.14; 12 months: 0.5 ± 0.33, p = 0.01).
After procedure, 11 eyes (22%) presented loss of BCVA of at least 2 lines. Of these, four eyes (8%) were cases of transient visual impairment, with spontaneous reversal by 3–6 months of follow-up. The remaining seven eyes (14%) maintained visual loss by months 6–12 of follow-up: five had advanced glaucoma and required additional procedures (trabeculectomy or tube surgery) for uncontrolled IOP and/or disease progression; the other two were cases of terminal glaucoma, with low vision at baseline (“counting fingers” or worse), that ultimately lost light perception. In total, five patients lost light perception: four were cases of terminal glaucoma with low vision at baseline (“counting fingers” or worse), and one occurred in the setting of severe hypotony described next.
Anisocoria, cataract progression, foreign body sensation, hyporeactive iris, mild inflammation, presbyopia and transient corneal oedema were recorded as adverse events, mostly transient and mild in nature. Eleven eyes (22.4%) presented with anisocoria (with treated pupil larger), of which nine were mild and transient (82%). One case of severe hypotony was registered, in a uveitis patient with Behçet disease. Procedure-related adverse events are detailed in Table 4. Four patients reported ocular pain during or after the procedure.
Discussion
Our study supports the HIFU cyclocoagulation of the ciliary body as a therapeutic option for adult patients with uncontrolled IOP despite optimised medical therapy. During the 1-year follow-up, mean IOP reduction from baseline was of 34%, and only eight patients (16.3%) additionally needed trabeculectomy or tube surgery during the study time span. Our findings are supported by Denis et al. [18], who assessed two different durations of HIFU cyclocoagulation (4 and 6 s) in patients with POAG and secondary refractory glaucoma. For both study groups, IOP respectively decreased 32% and 36% from baseline to 1-year post procedure, which is in line with our results.
Aptel et al. (EyeMUST1 Study; [19]) also investigated the efficacy of HIFU cyclocoagulation in the setting of refractory POAG. At 1-year post procedure, IOP-reductions from baseline were superior to 20% in 68% of included eyes. These steady results support the advantage of this technique, which efficacy has been suggested to further increase with repeated procedures [20].
As for the technical difficulty of this procedure, we can only comment on its user-friendly profile and fast procedure characteristics. Performing HIFU in cases with previous glaucoma surgery was uneventful. Although theoretically the ledge of tubes or blebs could difficult stablishing the vacuum, this was an easily overcome obstacle, and no procedure was cancelled, aborted or repeated for technical difficulties.
Regarding safety aspects, most adverse events were mild and transient. However, as a significant report, five patients experienced loss of light perception, one of which in the setting of severe hypotony in a young patient with a Behçet disease and ocular inflammatory manifestations. The EyeMUST1 Study included three patients with uveitic glaucoma, and no cases of hypotony were recorded in these patients’ study group. Nonetheless, our reports regarding visual acuity raise awareness to the importance of careful patient selection.
Our results additionally suggest patients submitted to HIFU may also experience relative BCVA impairment. While sustained decrease of BCVA may be explained through disease and/or cataract progression, the mechanisms underlying transient visual impairment are unclear. By revision of recorded adverse events, it is possible that pupillary changes may play a role, such as anterior chamber inflammation—nonetheless, the true mechanisms to this effect should be further analysed in future studies. As for the final visual acuity improvement (12 months vs. baseline), it is probably explained by previous study failures (patients needing additional glaucoma surgery), which were not assessed in the final study period.
Our study was mostly limited by its pragmatic design, precluding us from analysing data from all included patients at last follow-up. However, as to meet ethical standards, this design was considered by the investigators as the most suitable for the treatment of refractory glaucoma patients, allowing for more flexibility in the adjustment of treatment schemes and rescue therapies. In addition, we opted for a non-comparative design; as this is a recent technology, and still mostly applied to severe and refractory cases, its efficacy and safety profile should be well stablished prior to advancing to comparative studies.
Considering our previous findings, we believe HIFU cyclotherapy can act on three main levels: reduction of IOP levels, reduction of IOP-lowering drugs, and reduction/retardation of invasive glaucoma surgery. Our findings support its role as a valuable tool for patients with glaucoma refractory to medical therapy.
Summary
What was known before
-
High-intensity focused ultrasound (HIFU) cyclocoagulation of the ciliary body is an effective therapeutic option for adult patients with uncontrolled IOP despite optimised medical therapy.
-
Previous studies report 1-year IOP reductions of 32–36%.
-
Efficacy has been suggested to further increase with repeated procedures [20].
What this study adds
-
This study is the largest prospective report on the 8-s probe of the EyeOP1 device.
-
Our results support the efficacy of the HIFU for non-controlled glaucoma patients. Nearly 70% of patients benefited from this technique.
-
One case of serious hypotony occurred in the setting of uveitic glaucoma, though previous studies stated the safety of HIFU in this context.
-
Patients submitted to HIFU may experience transient visual disturbances.
References
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
Blumberg D, Skaat A, Liebmann JM. Emerging risk factors for glaucoma onset and progression. Prog Brain Res. 2015;221:81–101.
Choi J, Kook MS. Systemic and ocular hemodynamic risk factors in glaucoma. Biomed Res Int. 2015;2015:141905.
Boland MV, Quigley HA. Risk factors and open-angle glaucoma: concepts and applications. J Glaucoma. 2007;16:406–18. https://doi.org/10.1097/IJG.0b013e31806540a1.
Sousa DC, Leal I, Nascimento N, Marques-Neves C, Tuulonen A, Pinto LA. Use of ocular hypotensive medications in Portugal: PEM study: a cross-sectional nationwide analysis. J Glaucoma. 2017;26:571–6.
Kolko M, Horwitz A, Thygesen J, Jeppesen J, Torp-Pedersen C. The prevalence and incidence of glaucoma in Denmark in a fifteen year period: a nationwide study. PLoS ONE. 2015;10:e0132048.
Quaranta L, Riva I, Gerardi C, Oddone F, Floriani I, Konstas AG. Quality of life in glaucoma: a review of the literature. Adv Ther. 2016;33:959–81.
Janz NK, Wren PA, Lichter PR, Musch DC, Gillespie BW, Guire KE, et al. The collaborative initial glaucoma treatment study: interim quality of life findings after initial medical or surgical treatment of glaucoma. Ophthalmology. 2001;108:1954–65.
King AJ, Fernie G, Azuara-Blanco A, Burr JM, Garway-Heath T, Sparrow JM, et al. Treatment of advanced glaucoma study: a multicentre randomised controlled trial comparing primary medical treatment with primary trabeculectomy for people with newly diagnosed advanced glaucoma—study protocol. Br J Ophthalmol. 2018;102:922–8.
Mastropasqua R, Fasanella V, Mastropasqua A, Ciancaglini M, Agnifili L. High-intensity focused ultrasound circular cyclocoagulation in glaucoma: a step forward for cyclodestruction? J Ophthalmol. 2017;2017:7136275.
De R Jr. Cryosurgery for the treatment of glaucoma. Trans Am Ophthalmol Soc. 1965;63:189–204.
Hamard P, Gayraud JM, Kopel J, Valtot F, Quesnot S, Hamard H. Treatment of refractory glaucomas by transscleral cyclophotocoagulation using semiconductor diode laser. Analysis of 50 patients followed-up over 19 months. J Fr Ophtalmol. 1997;20:125–33.
Vernon SA, Koppens JM, Menon GJ, Negi AK. Diode laser cycloablation in adult glaucoma: long-term results of a standard protocol and review of current literature. Clin Exp Ophthalmol. 2006;34:411–20.
Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V, Hunter R, et al. Selective laser trabeculoplasty versus eye drops for first-time treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393:1505–6.
Aptel F, Denis P, Rouland JF, Renard JP, Bron A. Multicenter clinical trial of high-intensity focused ultrasound treatment in glaucoma patients without previous filtering surgery. Acta Ophthalmol. 2016;94:e268–77.
Posarelli C, Covello G, Bendinelli A, Fogagnolo P, Nardi M, Figus M. High-intensity focused ultrasound procedure: the rise of a new noninvasive glaucoma procedure and its possible future applications. Surv Ophthalmol. 2019;64:826–834.
Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. 2009;247:137–42.
Denis P, Aptel F, Rouland JF, Nordmann JP, Lachkar Y, Renard JP, et al. Cyclocoagulation of the ciliary bodies by high-intensity focused ultrasound: a 12-month multicenter study. Investig Ophthalmol Vis Sci. 2015;56:1089–96.
Aptel F, Dupuy C, Rouland JF. Treatment of refractory open-angle glaucoma using ultrasonic circular cyclocoagulation: a prospective case-series. Curr Med Res Opin. 2014;30:1599–605.
De Gregorio A, Pedrotti E, Stevan G, Montali M, Morselli S. Safety and efficacy of multiple cyclocoagulation of ciliary bodies by high-intensity focused ultrasound in patients with glaucoma. Graefes Arch Clin Exp Ophthalmol. 2017;255:2429–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marques, R.E., Ferreira, N.P., Sousa, D.C. et al. High intensity focused ultrasound for glaucoma: 1-year results from a prospective pragmatic study. Eye 35, 484–489 (2021). https://doi.org/10.1038/s41433-020-0878-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0878-0