Abstract
Background/objective
To determine if treatment of exudative age-related macular degeneration (eAMD) using proton beam therapy (PBT) combined with intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy is safe and effective long term.
Subject/methods
Thirty eyes with newly diagnosed eAMD were enrolled in a phase I/II prospective, sham-controlled double-masked university study. Eyes were randomized 1:1:1–24 GyE, 16 GyE or sham radiation, and treated with three initial monthly intravitreal ranibizumab or bevacizumab. Subsequent anti-VEGF reinjection was based on monthly optical coherence tomography and examination for 2 years and standard of care thereafter.
Results
A total of 23 eyes completed 2-year study follow-up, of which 16 maintained monthly follow-up. Mean best-correct visual acuity (BCVA) at 2 years was similar among treatment groups (p > 0.05). The 24 GyE group required fewer anti-VEGF injections when compared with the sham group at 2 years (4.67 ± 1.9 vs 9.67 ± 3.5; p = 0.017). Extended follow-up (mean 4 years) available in 22 eyes showed persistent reduced need for anti-VEGF therapy among eyes treated with 24 GyE compared with sham radiation (2.0 ± 1.6 vs 4.84 ± 2.4 per year, p = 0.008). New and increasing geographic atrophy (GA), noted in some eyes in all treatment groups, resulted in decreased mean BCVA from baseline for the 24 GyE group on extended follow-up (p = 0.009). Possible mild radiation retinopathy noted in 15% of eyes was not visually significant.
Conclusions
Initial treatment combining PBT (24 GyE) with intravitreal anti-VEGF therapy appears to decrease the need for anti-VEGF reinjection in eyes with newly diagnosed eAMD. Radiation retinopathy risk was low and does not appear visually significant. Long-term vision was limited by GA development especially in the 24 GyE group.
Similar content being viewed by others
Introduction
Exudative age-related macular degeneration (eAMD) remains a leading cause of legal blindness in the aging population [1, 2]. Intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy has improved visual prognosis, but prolonged retreatment is needed to maintain the benefit [3]. In order to minimize retreatment burden, various forms of supplemental radiotherapies have been explored [4,5,6,7,8,9]. Radiation may reduce the growth of fibrovascular tissue by destroying proliferating endothelial cells. Although ineffective as a monotherapy, synergistic effects may occur when combined with anti-VEGF therapy.
Proton beam therapy (PBT) provides a nonsurgical method of delivering radiation precisely to the macula [4,5,6,7]. Over 90% of radiation delivered via PBT is applied to the targeted tissue with minimal radiation dose to surrounding normal tissue [10, 11], thus minimizing risk of radiation retinopathy and papillopathy. It has been used to treat intraocular tumors with excellent results and good safety profile [10, 11]. A small pilot study showed no safety concerns combining 24 GyE PBT with intravitreal anti-VEGF therapy for eAMD [7]. Eyes with newly diagnosed eAMD required minimal retreatment with anti-VEGF therapy after PBT. Based on these observations, a larger randomized prospective, double-masked, sham-controlled study was conducted to further evaluate PBT combined with intravitreal anti-VEGF therapy. The 1 year interim analysis showed a synergistic effect using 24 GyE PBT, resulting in a significant reduction in the number of anti-VEGF retreatments [12]. The purpose of the current study was to test the hypothesis that PBT combined with intravitreal anti-VEGF therapy is safe and effective long term in treating eyes with eAMD.
Materials and methods
This study was conducted at The University of California Davis Eye Center under an IND cleared by the Food and Drug Administration (IND 108,360) and according to a protocol approved by the Office of Human Research Protection and the Office of Radiation Safety at the University of California Davis. The PBT was completed at the Crocker Nuclear Laboratory at the University of California Davis by the radiation oncology team from the University of California San Francisco. The study was registered with www.clinicaltrials.gov (NCT01213082) on October 1, 2010, before starting enrollment. It was conducted in compliance with the Declaration of Helsinki and Health Insurance Portability and Accountability Act of 1996.
Study methodology details have been previously described [12]. Briefly, this is a prospective, randomized, sham-controlled study. All study participants were enrolled at the University of California Davis Eye Center if active eAMD was noted with sub- or juxta-foveal choroidal neovascular membrane (CNVM) on fundus fluorescein angiography (FA) and best-correct visual acuity (BCVA) 20/40–20/400. Newly diagnosed eAMD were preferred for enrollment. Exclusion criteria included other macular or optic nerve comorbidities, history of diabetes mellitus, or prior head/neck radiation.
All participants underwent a complete eye examination, fundus photography, FA and optical coherence tomography (OCT) at baseline and were randomized 1:1:1–24 GyE PBT, 16 GyE PBT, or sham radiation after obtaining informed consent. Randomization was based on sequential coin tosses performed by an unmasked study coordinator. The initial anti-VEGF injection (ranibizumab 0.5 mg or bevacizumab 1.25 mg in 0.05 ml) was administered at enrollment with two additional monthly reinjections. Sham or PBT was administered within 6 weeks of enrollment in two fractionated doses 24 h apart. The participant and treating ophthalmologist were masked to randomization until study exit. The radiation oncologist was unmasked.
Participants were seen monthly for 24 months with dilated fundus examination and spectral-domain OCT imaging (SD-OCT, Cirrus, Carl Zeiss Meditec, Inc, Dublin, CA). Anti-VEGF reinjections were given monthly for persistent fluid until OCTs were stable. Thereafter, reinjection was for recurrent or increased intraretinal or subretinal fluid (SRF) on OCT or new or increasing macular hemorrhage during monthly visits. Central macular thickness (CMT) was obtained from the central 1 mm zone of the macula of the ETDRS macular thickness map.
Fundus photography and FA were repeated at 1 and 2 years when possible. The size of geographic atrophy (GA) was determined by measuring the area of involvement in relative disc areas noted on fundus photography and FA and confirmed by OCT. Choroidal neovascular membrane size was determined by measuring the area of early hyperfluorescence (including any rim of hypofluorescence) for classic lesions, area of late leakage on FA for occult lesions, and area involving the PED and any associated leakage for fibrovascular PEDs. The extent and intensity of leakage was graded on a scale of 0–3 (0 = no leakage, 1 = mild, 2 = moderate, and 3 = severe). All measurements were performed by a masked grader (LKM) and confirmed by a masked investigator (SSP). The rate of growth of GA was calculated by calculating the change in square root of GA area [13], averaged over the 2-year follow-up.
Most participants who completed the 24 monthly study follow-up continued standard of care treatment with the study investigators, receiving anti-VEGF retreatment based on treat and extend or as needed with monthly monitoring. The duration of these follow ups and final eye examination findings were recorded including BCVA, total number of anti-VEGF treatments received and presence of any vasculopathy suggestive of radiation retinopathy. The primary outcome measures were BCVA and number of anti-VEGF injections received at 24 months and adverse ocular events associated with radiotherapy at 24 months and last follow-up. Secondary outcome measures included size of GA, size and leakage of CNVM on FA and CMT on OCT at 24 months compared with baseline and BCVA and number of anti-VEGF injections received at last follow-up.
Proton beam and sham radiation
The 67.5 MeV proton beam extracted from the 76-inch isochronous cyclotron at Crocker Nuclear Laboratory, University of California Davis was used to treat participants using a protocol previously described [7, 12]. EYEPLAN V3.06 was used for treatment planning which registers the fundus or FA image as background image to delineate the target volume [14,15,16]. A 2-mm margin was placed around the projection of the lesion in the beam’s-eye-view.
Each participant’s head was immobilized with a thermoplastic mask in a head mask holder attached to the chair head holder. The immobilization of the subject’s eye was achieved by voluntary fixation of the gaze on a flashing light-emitting diode mounted on an azimuthal arm. The eyelids were kept open by using lid retractors after administering topical anesthesia. The eye was monitored closely using a video monitor during treatment. For sham radiation, the treatment steps were identical, except no proton beam treatment was delivered.
Statistical analysis
A sample size of 30 was chosen to allow for study enrollment in a reasonable time. This sample size was estimated to be large enough to detect differences among study groups based on chi-square analysis and observations from the pilot study showing only 25% requiring any further anti-VEGF treatment during the first year [7]. This is in contrast to published larger monotherapy PRN treatment studies where about 80–85% required additional anti-VEGF treatments [3, 17].
An estimate of variance was determined for each group of data and found to be similar between groups. A 2-tailed t-test was used to compare means. ANOVA testing was used to compare changes between treatment groups. Pearson Chi-square tests were used to determine differences in proportions for categorical data. P value < 0.05 was considered significant. The center values were calculated as mean + standard deviation.
Results
A total of 30 participants (30 eyes) were enrolled in the study between October 2010 and January 2015. For safety analysis, available follow-up information in all 30 study eyes was used. A total of 23 participants maintained ≥2 years follow-up, with 16 participants maintaining monthly follow-up until study exit. This cohort of 16 eyes, all with newly diagnosed eAMD at enrollment, was used for efficacy analysis (e.g. BCVA, number of anti-VEGF treatments). Seven participants lost to follow-up at 2 years included one who died and six excluded in the interim 1 year analysis for poor follow-up [12].
Table 1 summarizes the baseline characteristics of the 30 enrolled participants, the 23 participants completing ≥2 year follow-up and the 16 participants who completed monthly follow-up for at least 2 years. The three groups were similar. When these three groups of participants were further subdivided by study treatment, no significant difference was noted among groups except for the 16 GyE group where the baseline BCVA of the total enrolled cohort was worse than that of the subgroups that completed 2 years monthly follow-up (p = 0.03).
Visual acuity
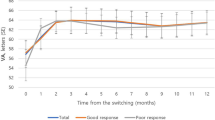
Mean BCVA at baseline for the cohort with 2 years follow-up (n = 23) was logMAR 0.64 (Snellen 20/90) with no significant difference among the three study groups (p = 0.174, Fig. 1a). Mean BCVA was unchanged at years 1 and 2 compared to baseline (Fig. 1a). There was no significant difference in mean BCVA among the three treatment groups at 1 or 2 years (p = 0.671, 0.197, respectively) although the mean BCVA of the 24 GyE group trended lower than baseline at 2 years (p = 0.05).
Mean best-corrected visual acuity of study groups at baseline, 1 year, 2 years and last follow-up for participants who maintained 2-year study follow-up (n = 23; a) and whose who maintained monthly study follow-up for 2 years (n = 16; b). P values shown are compared with baseline (BL) using a two-tailed Student t test unless otherwise specified. P < 0.05 is statistically significant difference from baseline.
For the 16 participants (16 eyes) that maintained monthly follow-up for 2 years, the mean BCVA for this cohort improved from baseline at year 1 (p = 0.02) but was not significantly different from baseline by year 2 (logMAR 0.58, Snellen–20/76; p = 0.47, Fig. 1b). There was no significant difference in the mean BCVA among the three treatment groups at baseline, 1-year and 2-year follow-up (Fig. 1b).
Anti-VEGF injections
Ranibizumab was the main anti-VEGF agent used in this study, with a few eyes receiving bevacizumab for insurance reasons (aflibercept was not used till study exit since not FDA-approved at study initiation). Among the 23 participants seen at 2-year follow-up, the 24 GyE PBT group received significantly fewer anti-VEGF injections than sham-treated eyes at 1 and 2 years (3.10 vs 6.38 at year 1 and 4.10 vs 10.25 at year 2, p < 0.001 and 0.002, respectively; Fig. 2a).
Mean number of intravitreal anti-VEGF injections administered at year 1 and 2 for the cohort maintaining 2-year study follow-up (a; n = 23) and subgroup with that maintained monthly study follow-up for 2 years (b; n = 16). Mean number of intravitreal anti-VEGF injections per year based on treatment group during post-study period (striped) and total follow-up period (solid) for the total cohort seen at study center after study exit (c; n = 22). P values shown are compared with the sham radiation group using a two-tailed Student t test. An asterisk denotes statistical significance (P < 0.05).
Similarly, among 16 participants that maintained monthly follow-up for 2 years, eyes treated with 24 GyE PBT received significantly fewer anti-VEGF injections compared with eyes treated with sham radiation (3.5 vs 6.5 at year 1; 4.67 vs 9.67 at year 2; p = 0.006 and p = 0.017, at year 1 and 2, respectively, Fig. 2b). Eyes treated with 16 GyE PBT had a trend toward fewer injections albeit not significant (4.75 vs 6.50, p = 0.198 at year 1; 7.25 vs 9.67; p = 0.531 at year 2, Fig. 2b).
Geographic atrophy (GA)
New or increasing GA was noted in some eyes in all three treatment groups in the region of the original CNVM at 2 years follow-up. The mean area of GA and the proportion of eyes with GA at baseline and at 2 years follow-up were not significantly different among treatment groups (ANOVA p = 0.542 at baseline and 0.193 at 2 years; Table 2). The calculated rate of growth of GA among study groups was not significantly different (p > 0.05). However, the 24 GyE PBT had a trend of having a larger mean GA size at baseline and higher rate of GA growth at 2 years follow-up when compared with the sham group.
Fluorescein angiography
All 23 eyes that maintained 2-year follow-up had FA at baseline with 14 of these eyes also having FA at year 2. The majority of CNVMs were classified as occult (16 of 23 eyes), with roughly equal distribution of this type of lesion among the three treatment groups. Among eyes with 2-year follow-up FA, the mean size of the CNVM at baseline and at 2 years follow-up for the study groups were not significantly different (p = 0.50 at baseline; p = 0.21 at 2-year, Fig. 3a). However, the two PBT groups show a trend toward reduction in mean CNVM sizes at 2-year follow-up while the opposite is noted for the sham radiation group (Fig. 3a).
a Mean size of choroidal neovascular membrane in disc areas at baseline and follow-up among eyes that had fluorescein angiography (FA) at 2 years for each study group. P value represents difference between groups; b Graph shows distribution of eyes in each study group with varying severity of leakage at baseline (left) and at 2 years follow-up (right). Leakage severity was graded as severe (3), moderate (2), mild (1), or none (0). P value represents difference between groups using ANOVA.
Similarly, the extent of leakage associated with the CNVM was graded numerically at baseline and 2-year follow-up (Fig. 3b). There was no difference in mean severity of leakage among the study groups at baseline and 2-year follow-up (p > 0.05). However, eyes in the 24 GyE group had the largest proportion of eyes with most severe leakage at baseline and the highest proportion of eyes without leakage at 2-year follow-up.
Optical coherence tomography (OCT)
Among eyes examined monthly till study exit at 2 years (n = 16), mean CMT decreased significantly from baseline at 1 year (p = 0.006) and 2 years (p = 0.006). The greatest decrease in mean CMT from baseline was noted in the 24 GyE group (−144 µm), followed by 16 GyE (−121 µm) and then sham (−37 µm) at 2 years, but the difference among groups was not statistically significant (p = 0.255). Although there was a trend toward a higher proportion of eyes being without intraretinal or subretinal fluid on OCT at 2 years follow-up in the PBT groups when compared with sham radiation group (60 and 80%, for 16 GyE and 24 GyE, respectively, compared with the sham group 25%), the difference was not significant (p = 0.064).
Extended follow-up
Twenty-two of the 23 eyes that completed 2-year follow-up continued care at the study center after study exit (mean 27 months; range 5–56 months). The mean follow-up time after study exit was not significantly different among the three study groups (p = 0.921). Although mean BCVA in the sham and 16 GyE groups at last follow-up was not significantly different from baseline (p = 0.96 and 0.40, respectively), the mean BCVA in the 24 GyE group was worse at last follow-up when compared with baseline (p = 0.009) due to GA development (Fig. 1a).
The mean number of additional anti-VEGF injections at last follow-up was highly variable (range 0–37) but the mean number of injections per year of follow-up over the entire study period and extended follow-up was significantly lower in the 24 GyE group compared with sham (2.0 ± 1.6 vs 4.84 ± 2.4, p = 0.008, Fig. 2c). A total of 5 of 23 eyes (all in 24 GyE cohort) no longer required additional anti-VEGF injections after study exit at 2-year follow-up.
Similarly, among 15 of 16 participants who completed the 2-year monthly study follow-up and continued care at the study center after study exit, the mean follow-up after exiting from the study was 26 months (range 5–56 months) with no difference among the study groups (p = 0.60). The mean BCVA of this subgroup of 15 participants at last follow-up showed improvement from baseline for the sham group (p = 0.02) but worsening for the 24 GyE group (ANOVA p value = 0.008) due to worsening GA limited to the region of the original CNVM lesion (Fig. 1b).
Safety considerations
All 19 of 30 participants that received 16 or 24 GyE PBT were included in the analysis of safety, even if they did not complete the monthly 2-year follow-up. No eye experienced severe visual loss (loss of >15 letters) within 2 years. After study exit, one eye in the 24 GyE cohort dropped from baseline BCVA of 20/60 to 20/70 at 2 years, then to 20/200 at 4 years of follow-up due to GA. However, the contralateral eye also developed GA on anti-VEGF monotherapy.
There was no eye with vision loss from radiation retinopathy or papillopathy during the study follow-up. Three eyes (15%; 1 in 16 GyE; 2 in 24 GyE) developed few transient retinal hemorrhages, cotton wool spots or mild retinal microvascular changes on FA likely from mild radiation retinopathy between 1 and 2 years after enrollment. No eye developed CME or ocular neovascularization due to radiation retinopathy.
There was no progression of cataract attributed to PBT. All eyes with cataract progression during follow-up had moderate cataracts at study enrollment.
Discussion
Based on the findings of this phase I/II prospective, sham-controlled trial, treatment of newly diagnosed eAMD with intravitreal anti-VEGF therapy combined with 24 GyE PBT appears to reduce the need for anti-VEGF reinjections at 1-year and 2-year follow-up. The effect might last beyond but our extended follow-up analysis should be interpreted with some caution since the retreatment regimen and anti-VEGF drug used changed in some eyes after study exit.
The current study provides the longest follow-up for eyes with eAMD treated with PBT and anti-VEGF therapy which is important for safety analysis (mean total follow-up >4 years). Although onset of radiation retinopathy is typically one to three years after radiation, visually significant retinopathy can be delayed [18]. In our study, 3 of 19 eyes that received PBT (15%) developed mild transient retinal vascular changes that likely represent mild radiation retinopathy; none were visually significant. Prior study using the same dose and fractionation of PBT as monotherapy for eAMD showed similar rate of mild radiation retinopathy after 2 years [19]. A few eyes had cataract progression in our study, but the rate was as expected for the natural progression of senile cataract.
A finding of note in our study is the incidence of GA in all treatment groups that appear to increase in size during follow-up (Table 2). Although the rate of GA growth was not statistically significant among treatment groups, there was a trend toward a higher rate in PBT treated groups, especially the 24 GyE cohort. It should be noted that the 24 GyE group also tended to have a higher mean size of GA at baseline although not significantly different from sham. On extended follow-up, GA was associated with decreased mean BCVA in the 24 GyE group. Progression of GA and associated vision loss has been observed in eyes with eAMD treated with anti-VEGF monotherapy after ≥2 years [3, 20]. Factors associated with GA formation in eyes with eAMD treated with anti-VEGF monotherapy include worse baseline BCVA, large CNVM size, intraretinal fluid, and GA in the fellow eye [13]. In our study, it is difficult to discern if radiation can accelerate GA progression. Trikha et al conducted a 10-year retrospective study of eyes treated with external beam radiation as monotherapy for eAMD and found a high incidence of GA that developed in the location of the original CNVM seen on pretreatment FA [21]. Since the size of the GA did not correlate with the size of the much larger radiation treatment field, it was felt that the GA resulted from the natural course of progression of eAMD and not from radiation. Similarly, in our study, GA developed only in the areas of the initial CNVM; areas within the radiation field but outside CNVM did not become atrophic during follow-up.
A limitation of this current study is the small sample size that may have limited our ability to detect subtle differences among study groups. The sample size for efficacy analysis was further decreased after excluding subjects not maintaining monthly follow-up. Despite this, a significant reduction in the number of anti-VEGF retreatment was noted in the 24 GyE group when compared with the sham radiation group. This finding is unlikely due to selection bias since the baseline characteristics of the subgroup used for efficacy analysis were similar to the total enrolled cohort.
An additional potential study limitation is the lack of standardization of anti-VEGF agent used during the study and after study exit. Either bevacizumab or ranibizumab was used during the study since the CATT study demonstrated no difference in visual acuity outcome at 2 years with either drug [3]. The majority of study participants received ranibizumab. After study exit, the choice of anti-VEGF therapy and the dosing regimen changed occasionally depending on patient and provider preference but were similar among the study groups.
In summary, this phase I/II study demonstrated that 24 GyE PBT reduced the need for anti-VEGF reinjections in eyes with newly diagnosed eAMD without major safety concerns associated with radiotherapy. Similar synergism was reported short-term using other radiation modalities, but vision was compromised at 2 years in the open-labeled prospective CABERNET study that used epiretinal brachytherapy, presumably from cataract progression after vitrectomy [8]. These alternative modes of radiation delivery have some limitations in targeting radiation precisely in the region of CNVM, a limitation which is minimized using PBT [7]. In our study, a customized treatment plan was made for each study eye using FA, fundus photograph and axial length, analogous to that used successfully to treat ocular melanoma [22]. Using this approach, no adverse effect on vision from cataract progression or radiation retinopathy was noted, but GA was noted at baseline in some eyes with growth on follow-up which limited vision long term especially in the 24 GyE group. Since GA progression is a well-documented long-term finding in eyes with eAMD treated with anti-VEGF monotherapy, this likely represents the natural course of progression of AMD. Whether radiation can contribute to progression of GA is unclear based on the results of our small study. A larger long-term study would be useful to fully assess the effect of PBT combined with intravitreal anti-VEGF in treating eAMD.
Summary
What was known before
-
Low dose radiation, including PBT, is relatively safe but ineffective as monotherapy for eAMD.
-
Intravitreal anti-VEGF monotherapy minimizes vision loss associated with eAMD but requires frequent re-administration. Eyes on long term anti-VEGF monotherapy for eAMD can develop GA long term that limits vision.
-
Radiation combined with anti-VEGF in eyes with newly diagnosed eAMD decreases the need for anti-VEGF injection for at least 1 year, but long-term effects were unknown.
What this study adds
-
Low dose proton beam combined with intravitreal anti-VEGF injection results in reduced need for reinjection with anti-VEGF drugs in eyes with newly diagnosed eAMD for at least 2 years and possibly beyond.
-
Mild radiation retinopathy may be observed when combining intravitreal anti-VEGF with proton beam for eAMD but radiation retinopathy was not visually significant.
-
On extended follow-up, GA developed in the area of original CNVM in some eyes with eAMD that limited long-term vision, especially in the group treated with 24 GyE proton beam.
References
Bourne R, Jonas J, Bron A, Cicinelli MV, Das A, Flaxman SR, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: magnitude, temporal trends, and projections. Br J Ophthalmol. 2018;102:575–85.
Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e1234.
Maguire MG, Martin DF, Ying GS, Jaffe GJ, Daniel E, Comparison of Age-related Macular Degeneration Treatments Trials Research Group, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123:1751–61.
Ciulla TA, Danis RP, Klein SB, Malinovsky VE, Soni PS, Pratt LM, et al. Proton therapy for exudative age-related macular degeneration: a randomized sham-controlled clinical trial. Am J Ophthalmol. 2002;134:905–6.
Yonemoto LT, Slater JD, Friedrichsen EJ, Loredo LN, Ing J, Archambeau JO, et al. Phase I/II study of proton beam irradiation for the treatment of subfoveal choroidal neovascuarlization in age-related macular degeneration: treatment techniques and preliminary results. Int J Radiat Oncol Biol Phys. 1996;36:867–71.
Flaxel CJ, Friedrichsen EJ, Smith JO, Oenick SC, Blacharski PA, Garcia CA, et al. Proton beam irradiation of subfoveal choroidal neovascularization in age-related macular degeneration. Eye (Lond). 2000;14:155–64.
Park SS, Daftari I, Phillips T, Morse LS. Three-year follow-up of a pilot study of ranibizumab combined with proton beam irrradiation as treatment for exudative age-related macular degeneration. Retina. 2012;32:956–66.
Dugel PU, Bebchuk JD, Nau J, Reichel E, Singer M, Barak A, et al. Epimacular brachytherapy for neovascular age-related macular degeneration: a randomized, controlled trial (CABERNET). Ophthalmology. 2013;120:317–27.
Brand C, Arnoldussen M. IRay therapy as an adjuvant therapy in newly diagnosed patients with neovascular age-related macular degeneration. Eye (Lond). 2018;32:1345–52.
Bensoussan E, Thariat J, Maschi C, Delas J, Schouver ED, Hérault J, et al. Outcomes after proton beam therapy for large choroidal melanomas in 492 patients. Am J Ophthalmol. 2016;165:78–87.
Sikuade MJ, Salvi S, Rundle PA, Errington DG, Kacperek A, Rennie IG. Outcomes of treatment with stereotactic radiosurgery or proton beam therapy for choroidal melanoma. Eye (Lond). 2015;29:1194–8.
Osmanovic S, Moisseiev E, Mishra KK, Daftari I, Moshiri A, Morse L, et al. Phase I/II randomized study of proton beam with anti–vascular endothelial growth factor for exudative age-related macular degeneration. Ophthalmol Retin. 2017;1:217–26.
Grunwald JE, Pistilli M, Daniel E, Ying GS, Pan W, Jaffe GJ, et al. Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology. 2017;124:97–104.
Daftari I, Mishra KK, Singh RP, Shadoan DJ, Phillips TL. An overview of the control system for dose delivery at the UCSF dedicated ocular proton beam. Int J Med Phys Clin Engg Rad Oncol. 2016;5:242–62.
Goitein M, Miller T. Planning proton therapy of the eye. Med Phys. 1983;10:275–83.
Daftari I, Mishra KK, O'Brien JM, Tsai T, Park SS, Sheen M. Fundus image fusion in EYEPLAN software: an evaluation of a novel technique for ocular melanoma radiation treatment planning. Med Phys. 2010;37:5199–207.
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO study. Am J Ophthalmol. 2009;148:43–58 e1.
Guyer DR, Mukai S, Eaga KM, Seddon JM, Walsh SM, Gragoudas E. Radiation maculopathy after proton beam irradiation for choroidal melanoma. Ophthalmology. 1992;99:1278–95.
Zambarakji HJ, Lane AM, Ezra E, Gauthier D, Goitein M, Adams JA, et al. Proton beam irradiation for neovascular age-related macular degeneration. Ophthalmology. 2006;113:2012–9.
Bhisitkul RB, Mendes TS, Rofagha S, Enanoria W, Boyer DS, Sadda SR, et al. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. Am J Ophthalmol. 2015;159:915–924 e2.
Trikha R, Morse LS, Zawadzki RJ, Werner JS, Park SS. Ten-year follow-up of eyes treated with stereotactic fractionated external beam radiation for neovascular age-related macular degeneration. Retina. 2011;31:1303–15.
Gragoudas ES. Proton beam irradiation of uveal melanomas: the first 30 years. The Weisenfeld Lecture. Invest Ophthalmol Vis Sci. 2006;47:4666–73.
Funding
The study was supported in part by the Strategic Opportunities Support Award, Clinical and Translational Science Institute, University of California San Francisco.
Author information
Authors and Affiliations
Contributions
All authors met criteria for authorship by making substantial contributions to conception or design of work, acquisition, analysis, or interpretation of data, or in drafting or revising paper critically for important intellectual content. All authors approved the final version of this work.
Corresponding author
Ethics declarations
Conflict of interest
SSP has received research support via employer from Allergan and Roche/Novartis for contracted clinical research exploring anti-VEGF drugs for treatment of retinal disorders including exudative AMD. None of the other authors have any conflict of interest to disclose.
Disclosure
Contracted research with Allergan and Roche Novartis via employer (SSP) investigating anti-VEGF therapy for retinal disorders, including macular degeneration. No other conflicting relationship exists for any of the remaining authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mukkamala, L.K., Mishra, K., Daftari, I. et al. Phase I/II randomized study of proton beam with anti-VEGF for exudative age-related macular degeneration: long-term results. Eye 34, 2271–2279 (2020). https://doi.org/10.1038/s41433-020-0807-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0807-2