Abstract
Background/objectives
Abnormal retinal neovascularization caused by ischemic retinal vein occlusion (RVO) is a frequent cause of visually significant vitreous hemorrhage. The early detection of new vessels may be challenging and often requires the use of invasive tests such as fundus fluorescein angiography (FA). We demonstrate the use of wide-field optical coherence tomography angiography (WF-OCTA) in the detection and characterization of neovascularization secondary to ischemic RVO.
Subjects/methods
We conducted a retrospective observational case series of patients diagnosed with ischemic RVO between August 2018 and March 2019, who underwent WF-SS-OCTA imaging (PLEX Elite 9000, Carl Zeiss Meditec). We performed real-life montage imaging, covering the involved area and compared the findings of WF-SS-OCTA to standard clinical examination and when available, ultrawide-field fluorescein angiography (UWF-FA, Optos 200TX).
Results
In the included 39 eyes with ischemic RVO, neovascularization elsewhere (NVE) was encountered in 16 of 39 eyes (41%) on WF-OCTA and were characterized as sea-fan type vessels and nodular type vessels, based on their appearance and localization. NVE was identified in 4/39 eyes on standard clinical examination, equating to a detection rate of 10.3%. All were of a sea-fan morphology. In one case, NVE found on WF-OCTA was not observed on UWF-FA, which was a nodular type. Neovascularization of the disc (NVD) was detected in one eye.
Conclusions
WF-OCTA may become a useful noninvasive tool in the detection of neovascularization in patients with ischemic RVO. Furthermore, the characterization of different morphologies of neovascularization detected by WF-OCTA could be of clinical relevance
Similar content being viewed by others
Introduction
Retinal vein occlusion (RVO) is a common retinal vascular disease and cause for sight loss [1]. To evaluate the severity of the disease, including classification by location, amount of ischemia or macular edema, retinal examination and imaging are mandatory. Traditionally, fluorescein angiography (FA) was used to evaluate the degree of ischemia and capillary dropout, as well to detect neovascularization [2]. Optical coherence tomography (OCT) has become a crucial imaging modality providing for structural analysis of the retina [3]. OCT angiography (OCTA) has also emerged as a novel modality to visualize the retinal vasculature detecting differences in amplitude, intensity or phase variance between sequential B-scans. However, its small field has been a limiting factor [4].
OCTA detects macular ischemia and, with the development of wide-field OCTA, more peripheral areas of nonperfusion (ANP) in the distribution of the occluded vein can also be evaluated [5, 6]. Similarly, wide-field OCTA has been shown to be valuable for analysis of the retinal vasculature and early detection of neovascularization (NV) in proliferative diabetic retinopathy (PDR) [7, 8]. This is of particular clinical interest as OCTA is a noninvasive and safely repeatable test compared to FA, which is frequently accompanied by nausea and rarely potential anaphylactic responses to the dye [9]. Panretinal photocoagulation (PRP) is the mainstay of treatment to prevent complications secondary to the development of abnormal blood vessels and vitreous hemorrhage by inducing vessel regression. Early detection of neovascularization is paramount, as it has been shown in two landmark studies that scatter laser treatment should only be applied after the occurrence of neovascularization [10, 11]. This is further complicated by the fact that nonischemic RVO can convert into ischemic RVO, hence repeated clinical observation is essential in order to prevent complications [10, 12, 13]. With the development of high speed wide field swept source OCTA (WF-OCTA) capturing 12 × 12 mm or 15 × 9 mm scans with eye tracking and montage of five images, ischemic retinal areas can be directly imaged. The aim of this study is to demonstrate the use of WF-OCTA in phenotyping and hence, the early detection of neovascularization after ischemic RVO.
Materials and methods
Data collection
This study is a retrospective observational case series including patients diagnosed with branch retinal vein occlusion (BRVO) hemiretinal vein occlusion (HRVO) or central retinal vein occlusion (CRVO), clinically suspected to be ischemic, who underwent WF-OCTA imaging (PLEX Elite 9000, Carl Zeiss Meditec, AG) as a part of routine clinical care between August 2018 and March 2019. CRVO is defined as a thrombotic event that affects the central retinal vein as it passes the lamina, with disc swelling, venous tortuosity and hemorrhages in four quadrants cribrosa, HRVO affect either the superior or inferior hemisphere with nearly equally distributed hemorrhages in two altitudinal quadrants, a BRVO occurs at an arterio-venous crossing affecting the area drained by the affected vein [14]. Clinical signs of ischemia documented in the medical records included poor visual acuity, multiple deep intraretinal hemorrhages, cotton wool spots, the degree of venous dilation and tortuosity, the presence of a relative afferent pupillary defect in cases with CRVO and the amount of nonperfused areas on FA if available, at presentation [14]. The initial clinical diagnosis (used in this study) was made prior to OCTA acquisition and was documented in the patient clinical notes. Following the diagnosis, and based on clinical suspicion, OCTA was requested and its results are documented in the study. We excluded patients with severe media opacities limiting a clear scan, if the obtained OCTA scan did not image cover the affected area, if the ANP was less than 5 DA or when insufficient image quality precluded its assessment. Demographic and clinical data were collected for all patients. Clinical data included best-corrected visual acuity (BCVA), ultrawide-field color fundus photography (UWF-CFP), B-scan (structural) OCT, and FA in selected cases. Approval for data collection and analysis was obtained from the Institutional Review Board at Moorfields (ROAD17/031). The study adhered to the tenets set forth in the Declaration of Helsinki.
Image acquisition protocol
OCTA scans were obtained using a PLEX Elite 9000, Carl Zeiss Meditec. Briefly, a swept-source tuneable laser with a wavelength of 1040 nm and a scanning rate of 100,000 A-scans per second provided a transverse resolution of 20 μm and an optical axial resolution of 6.3 μm (in tissue) with a digital axial resolution of 1.95 μm (in tissue). The instrument has an A-scan depth of 3.0 mm in tissue (1536 pixels). The 12 x 12 mm scans apply 500 A-scans per B-scan at 500 B-scan positions, resulting in an A-scan and B-scan separation of 24 μm. Two sequential B-scans are performed at each fixed position before proceeding to the next transverse location on the retina.
The scanning protocol used was 12 × 12 OCTA Montage, in which five 12 × 12 mm OCTA data cubes positioned at different locations (superotemporal, superonasal, inferotemporal and inferotemporal) were acquired. WF-OCTA en face montage was automatically generated with a total field of view up to 80°. Figure 1 shows an example of the 12 × 12 OCTA Montage in a patient with BRVO. In some patients 12 × 12, 15 × 9 or 9 × 9 were applied. A given scanning protocol was selected to cover almost all the area affected by the occluded vein.
The en face SS-OCTA images visualized the flow that was detectable within a volume defined by selected boundaries. Each of these segmented volumes is referred to as a slab. To detect NV, the vitreoretinal interface (VRI) segmentation and superficial retinal segmentation were used. The superficial retinal slab was defined as the volume between the internal limiting membrane (ILM) and the outer boundary of the inner plexiform layer (IPL). The vitreoretinal interface (VRI) slab was defined with an inner boundary 300μm above the ILM and an outer boundary at the ILM. Where available, FA photographs, when available, were acquired using the widefield (WF) Optos 200TX (Optos Plc, Dunfermline, Scotland).
Image grading
Two masked, trained retina specialists (HK, JH) independently reviewed the structural B-scans with overlaid flow signal, and the en face OCTA images of the superficial capillary plexus and VRI in each cube of the OCTA as well as the UWF-FA images when available.
Areas of nonperfusion
ANP were graded on the en face full retinal thickness slab OCTA similar to the clinical interpretation of FA respecting the amount of nonperfusion in correlation to the disc areas on the image, as it was shown recently that OCTA can be used for quantification of retinal nonperfusion in BRVO [6]. The disc areas were measured from the individual using a circle adjusted manually according to the disc size. The threshold for ischemic branch retinal vein occlusions (BRVO) was five disc areas and ten disc areas for central retinal vein occlusions (CRVO) [10, 15]. Therefore, we categorized our cohort into two groups: limited ischemia with five to ten disc areas involved, and the extensive type with ten or more disc areas of ANP.
Neovascularization at the disc and elsewhere
Neovascularization at the disc (NVD) and neovascularization elsewhere (NVE) were identified as preretinal hyperreflective material in the OCT B-scan (PRHM) which was detected by careful assessment of each individual OCT B-scan within the volume; the flow signals within the PRHM were interpreted qualitatively as a sign suggesting activity of the new vessels [16]. This was correlated with en face OCTA images to delineate any abnormal vascular network at the level of the corresponding B-scan where the PRHM were detected.
Furthermore, NVE were sub stratified into sea-fan type vessels (Fig. 2), a neovascular network growing along the posterior hyaloid and nodular vessels (Fig. 3), which presented as small buds arising from large retinal vessels. The appearance of the neovascularization in relationship with the ANP was analyzed and compared to the UWF-FA, if available.
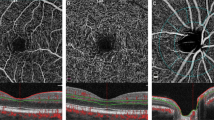
En face optical coherence tomography angiography (OCTA) of the superficial retinal plexus slab at the superonasal quadrant of 12 × 12 OCTA montage (a) demonstrating area of nonperfusion secondary to ischemic branch retinal vein occlusion with evident hyperreflective sea-fan like lesion at the edge of the ischemic area which is clearly seen also in the vitreoretinal interface slab (arrow) (b) and corresponds to the preretinal hyperreflective material scaffolding the posterior hyaloid with evident flow signals in the OCTA B-scan (arrow) (c).
Central 12 × 12 en face optical coherence tomography angiography(OCTA) of the superficial plexus (a) demonstrating area of nonperfusion and perivascular hyperreflective lesions (arrow) which could be seen also in the vitreoretinal interface slab (arrow) (b) and corresponds to the nodular preretinal hyperreflective material seen in B-scan OCTA with overlaid flow signals (arrow) (c).
For FA grading, dye leakage in areas suspected to be neovascularization by clinical examination and OCT was recorded. The findings from the WF-OCTA were compared to the standard clinical examination and the available data collected from UWF-CFP and UWF-FA. Disagreements between graders were adjudicated by a senior retina specialist (PAK).
Statistical analysis
Inter-rater agreement was calculated using kappa statistics, wherein a Kappa (K) value of less than 0.2 means poor agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 good agreement, and 0.81–1.00 very good agreement [17]. Statistical analyses were performed using MedCalc® version 19.0.5 (MedCalc Inc., Mariakerke, Belgium).
Results
A total of 60 eyes of 58 patients with retinal vein occlusion who have attended medical retina clinics in Moorfields Eye Hospital August 2018 and March 2019 and were imaged using WF-OCTA. Of these, 42 eyes of 40 patients were diagnosed clinically with ischemic RVO. Two eyes were excluded because of poor image quality secondary to poor fixation. One eye was excluded because the OCTA did not cover the ANP. Therefore, 39 eyes of 37 patients with ischemic RVO were included in the study. Of these, nine patients were diagnosed with CRVO, three patients with HRVO, and25 patients with BRVO, of which five eyes of three patients were secondary to systemic inflammatory disorders. The inter-rater agreement was 0.89 for neovascularization detection on OCTA, 1.0 for structural OCT, and 1.0 for UWF-FA.
Detection of neovascularization
WF-OCTA detected NVE in 16 of 39 eyes (41%), while the clinical examination only identified four eyes with NVE (10.3%). The flow signals were detected in the OCTA B-scan in 15 eyes (38.5%). Among the eyes with NVE observed on OCTA, fifteen eyes demonstrated extensive ANP (ten disc areas or more) and one eye limited ANP where ischemia involved five to ten disc areas. Of the 16 eyes with NVE, three had CRVO (18.75%), 13 eyes had BRVO (81.25%), of which, three BRVO were secondary to systemic inflammatory disorders (18.75%). Eight of these 16 eyes were previously treated - five of them with PRP two with anti-vascular endothelial growth factor (VEGF), and one with both PRP and anti-VEGF. NVD was detected in only one eye (Table 1).
Type of neovascularization elsewhere detected
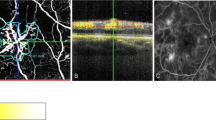
Twelve eyes (75%) showed NVE of a nodular type. Figure 3 displays an example of a nodular type vessels in the superior temporal arcade after BRVO. This was compared to four eyes (25%) with both nodular type vessels and sea-fan type vessels. A representative case is shown in Fig. 2. Interestingly, the four eyes with clinically detectable NVE consisted of sea-fan type vessels. Four patients had simultaneous UWF-FA. In all of them the NVs were detectable on OCTA. However, in one of four eyes where OCTA detected NVE, FA could not provide evidence of NVE despite the detection with OCTA; the neovascularization detected was of a nodular type vessel. Figure 4 shows the UWF-FA image with no signs of NVE whereas the corresponding en face OCTA provides evidence of PHRM with flow signal.
Ultrawide-field fluorescein angiography (UWF-FA) centered at the area involved with branch retinal vein occlusion demonstrating massive areas of nonperfusion (ANP) (a). En face optical coherence tomography angiography (OCTA) of the superficial retinal plexus slab at the superotemporal quadrant of 12 × 12 OCTA montage (b), covering the area involved by the occluded vein showing more evident ANP compared to fluorescein angiography(FA) and demonstrating perivascular Nodular type of neovascularization which could be detected in B-scan as preretinal hyperreflective material with overlaid flow signals (c) and hardly could be detected in FA (arrow).
Detection of areas of nonperfusion
Extensive ANP were detected in 32 eyes while limited ANP were found in seven eyes; of these seven, we found four eyes where approximately five disc areas were involved, three with macular BRVO and one with RVO secondary to systemic inflammatory disorders. Three eyes had scattered ANP between five and ten disc areas. Among the ten patients, who additionally had UWF-FA, there was 100% agreement on the ANP distribution within the fields visualized on both modalities.
Discussion
Whereas FA has traditionally been the gold standard modality to detect ANP and the development of neovascularization, WF-OCTA may provide a safe alternative to diagnose and monitor the complications of ischemic RVO. When the affected areas can be adequately visualized, clinicians may detect new vessels at earlier stages, monitor them without the potential risks of an invasive dye test.
In our study, we could demonstrate NVE in 41% of involved eyes, 19% of these with CRVO and 62.5% with BRVO with the use of WF-OCTA. To our knowledge, OCTA has not been used to describe neovascularization caused by ischemic RVO. Nevertheless, different groups have shown that neovascularization caused by proliferative diabetic retinopathy can be detected by OCTA [18, 19]. In ischemic RVO, NVE are more likely to be found in BRVO, occurring at the border of nonperfusion to perfusion areas; only occasionally are they found in the periphery [20, 21].
Using WF-OCTA, we could identify two different types of new vessels: sea-fan type vessels and nodular vessels. Interestingly, all four cases of sea-fan type vessels were detected in a standard clinical examination, whereas the nodular type vessels were detected with advanced imaging only. In regards to the location of these vessels, the sea-fan type vessels in our study were located close to the edge of ischemia, growing along the scaffolding of the posterior hyaloid. Nodular type vessels, on the other hand, were found originating from first and second degree veins and varied in their relationship to the borders of ischemic areas. This is, to our knowledge, the first time, these distinct forms of neovascularization in RVO have been described. Because of the nodular appearance and the unusual location, in our cohort, they were frequently misdiagnosed as retinal hemorrhage in clinical examination. Of interest is that we were able to describe one case, where on FA nodular type neovessels did not present with leakage, whereas the OCTA showed obvious NVE with evident flow signals suggesting their activity (Fig. 4). We would like to point out that this could be a false positive finding on OCTA. However, the ability of OCTA to detect all retinal layers in detail, the particular shape of the NVE on the B-scan with its location and the flow signal within the lesion though, was of similar structure as NVE described in the literature [7, 8]. Owing to its presence predominantly alongside first and second degree vessels, these subtle neovascular changes could be misdiagnosed as perivenous staining in FA which is a common finding in RVO [22]. In our cohort, we observed one case with NVD where the flow signal of the PHRM determined clinical activity. Although CRVO are more likely to develop NVD [23], in the eye with NVD detected the underlying pathology was a BRVO secondary to systemic inflammation.
We were able to detect NVE undetectable clinically, most of them of the nodular type. Our study raises the question of whether all types of new vessels detected after RVO should be treated, or if, in selected cases, careful clinical observation with the use of OCTA could be feasible. Natural history studies on RVO suggest that approximately 20% of patients with BRVO develop NV, of which 60-90% develop a vitreous hemorrhage [24, 25]. The BVOS landmark trial concluded that sectoral PRP in ischemic BRVO did not prevent the development of NV, but significantly reduced the risk of vitreous bleeding [10]. All these studies share the fact that they were conducted prior to the OCTA era, relying entirely on FA and careful clinical examination. With this novel insights, WF-OCTA can potentially detect more NV and might particularly be useful for clinical judgment of these cases, preventing further complications.
With the help of WF-OCTA, we were able to detect persistent neovascularization in eight eyes previously treated with either sectoral PRP or intravitreal anti-VEGF for macular edema, seven of them showing flow signals. This sheds light on the role of WF-OCTA as a clinical follow up modality for patients with treated ischemic RVO. Although, there is currently no conclusive definition for the activity of neovascularization on all imaging modalities, it is currently based on the assumption that the existence of blood flow within a neovascular complex designates it as active. It is possible that blood flow may remain in stable, mature complexes. One might argue that these nodular type NVE may or may not be malformations rather than NVE. When comparing these formations with other neovascularizations, the common feature is that all these NVs are to be found above the ILM, growing towards the vitreous space, which is a key element of neovascularizations in other ischemic retinal diseases. Malformations like collateral vessels tend to establish in a horizontal fashion unlike NVE and are therefore to be found intraretinal. Moreover, modern OCTA machines use eye-tracking devices and are more suitable for repeated examinations, comparing the blood flow and potential regression in due course. However, longitudinal studies to evaluate this will be necessary.
In our study, we show that WF-OCTA is able to identify ANP and analyze the amount of ischemia. We found extensive ANP with more than ten disc areas in 82 % of our cases on WF-OCTA and limited ANP of five to ten disc areas in 18% of eyes. Clinical distinction between ischemic and nonischemic RVO is of importance due to the potential development of NV [26]. Historically, the CVOS landmark trial defined the ischemic threshold as ten disc areas in CRVO, whereas the BVOS trial referred to an affected area of 5 disc diameters in BRVO as an ischemic threshold [10, 15]. The SCORE study however suggested that eyes with CRVO or BRVO and an ANP greater than 5.5 disc areas should be labeled as ischemic, as in CRVO 16.6 % of eyes developed neovascular glaucoma (NVG) versus 4.0% with ANP less than 5.5 disc areas after 36 months, and 14.6% vs 2.4% of BRVO eyes developed NVD/NVE respectively [27]. When comparing ten cases of our cohort with both FA and WF-OCTA, we could show 100% agreement between the two modalities. Interestingly, the underestimation of capillary density in FA has been shown to be less than 50% compared to histologically based assessments [28]. OCTA on the other hand has higher rates of detecting areas of nonperfusion as it can detect both the superficial and deep plexus, while FA can only display the superficial plexus [29]. Additionally, WF-OCTA has the advantage of visualizing a wider field of view, while commercially available OCTA are hindered by the limited scan area. Recently, stratification of the distribution of ischemia in CRVO has shown, that ischemia at the posterior pole is of significantly higher risk for developing NV than in the periphery [30]. This underpins the newly found significance of WF-OCTA in the assessment of RVO, especially at the posterior pole.
Limitations to our study include the small number of cases and the retrospective analysis. Furthermore, we cannot provide FA for all patients. Two patients had motion artefacts on their OCTA images despite the eye-tracking function. Additionally, there is a controversy about the definition of ischemic RVO. While an image based grading is widely used due to feasibility, some advocate a more extensive examination, including Goldmann visual fields or ERG to confirm the diagnosis [2]. No standardized clinical observation protocol was in place. Due to the retrospective design of our study, a number of our patients had been treated previously with intravitreal anti-VEGF injections or laser, hence neovascularizations might have regressed after treatment. Duration was calculated as the first visit to Moorfields to the time of NVE detection and might not represent the actual disease duration. Longitudinal studies to detect complications from early detected vessels would provide more insight into the natural history of RVO. It is still unclear whether the ability of OCTA to detect small neovascularisation not visible on conventional examination has clinical significance as this is not a part of current routine practice. Little is known about the risk of vitreous hemorrhage from nodular type vessels, as most of the research in this field has been undertaken prior to the area of modern imaging. Furthermore, the effect of PRP on the activity of NV could be analyzed, as until now this is firstly a matter of clinical experience and secondly dependent on the amount of repeated dye tests.
Our retrospective study demonstrates the value of WF-OCTA in RVO to detect early vessel development and describe different types of NV. We show that OCTA as a noninvasive, repeatable imaging modality has the potential to monitor patients with early proliferative disease and provide more patient centered care.
Summary
What was known before
-
Ischemic retinal vein occlusions can cause abnormal retinal neovascularization, a frequent cause of vitreous hemorrhage.
-
Early detection can be difficult and often requires fluorescein angiography.
What this study adds
-
Widefield OCTA can be used to detect and phenotype neovascularization.
-
We describe different types of neovascularization, based on appearance and location.
References
Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–9.e1.
Hayreh SS, Klugman MR, Beri M, Kimura AE, Podhajsky P. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefe’s Arch Clin Exp Ophthalmol. 1990;228:201–17. https://doi.org/10.1007/bf00920022.
Keane PA, Sadda SR. Retinal imaging in the twenty-first century. Ophthalmology. 2014;121:2489–2500. https://doi.org/10.1016/j.ophtha.2014.07.054.
Gao SS, Jia Y, Zhang M, Su JP, Liu G, Hwang TS. et al. Optical coherence tomography angiography. Investigative Opthalmol Vis Sci. 2016;57:OCT27–36. https://doi.org/10.1167/iovs.15-19043.
Cardoso JN, Keane PA, Sim DA, Bradley P, Agrawal R, Addison PK. et al. Systematic evaluation of optical coherence tomography angiography in retinal vein occlusion. Am J Ophthalmol. 2016;163:93–107.e6. https://doi.org/10.1016/j.ajo.2015.11.025.
Shiraki A, Sakimoto S, Tsuboi K, Wakabayashi T, Hara C, Fukushima Y, et al. Evaluation of retinal nonperfusion in branch retinal vein occlusion using wide-field optical coherence tomography angiography. Acta Ophthalmol. 2019;97:e913–8.
Hirano T, Kakihara S, Toriyama Y, Nittala MG, Murata T, Sadda S. Wide-field en face swept-source optical coherence tomography angiography using extended field imaging in diabetic retinopathy. Br J Ophthalmol. 2018;102:1199–203.
Sawada O, Ichiyama Y, Obata S, Ito Y, Kakinoki M, Sawada T, et al. Comparison between wide-angle OCT angiography and ultra-wide field fluorescein angiography for detecting non-perfusion areas and retinal neovascularization in eyes with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2018;256:1275–80.
Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W. et al. Fluorescein angiography complication survey. Ophthalmology. 1986;93:611–7. https://doi.org/10.1016/s0161-6420(86)33697-2.
The Branch Vein Occlusion Study Group. Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. Arch Ophthalmol. 1986; 104:34. https://doi.org/10.1001/archopht.1986.01050130044017.
Laatikainen L, Kohner EM, Khoury D, Blach RK. Panretinal photocoagulation in central retinal vein occlusion: a randomised controlled clinical study. Br J Ophthalmol. 1977;61:741–53. https://doi.org/10.1136/bjo.61.12.741.
Arsene S, Vierron E, Le Lez ML, Herault B, Gruel Y, Pisella PJ, et al. Conversion from nonischemic to ischemic retinal vein occlusion: prediction by venous velocity on color Doppler imaging. Eur J Ophthalmol. 2009;19:1009–16.
Minturn J, Brown GC. Progression of nonischemic central retinal vein obstruction to the ischemic variant. Ophthalmology. 1986;93:1158–62.
Royal College of Ophthalmology. RCOphth Retinal Vein Occlusion Guidelines. 2015. https://www.rcophth.ac.uk/wp-content/uploads/2015/07/Retinal-Vein-Occlusion-RVO-Guidelines-July-2015.pdf. Accessed April 2019.
The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischermic central vein occlusion. Ophthalmology. 1995;102:1434–44. https://doi.org/10.1016/s0161-6420(95)30848-2.
Schwartz R, Khalid H, Sivaprasad S, Nicholson L, Anikina E, Sullivan P, et al. Objective Evaluation of Proliferative Diabetic Retinopathy Using OCT. Ophthalmol Retina. 2019. https://doi.org/10.1016/j.oret.2019.09.004.
Brennan P, Silman A. Statistical methods for assessing observer variability in clinical measures. BMJ. 1992;304:1491–4.
de Carlo TE, Bonini Filho MA, Baumal CR, Reichel E, Rogers A, Witkin AJ, et al. Evaluation of preretinal neovascularization in proliferative diabetic retinopathy using optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina 2016;47:115–9.
Jia Y, Bailey ST, Hwang TS, McClintic SM, Gao SS, Pennesi ME, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci USA. 2015;112:E2395–402.
Finkelstein D, Clarkson J, Diddie K, Hillis A, Kimball A, Orth D, et al. Branch vein occlusion. Retinal neovascularization outside the involved segment. Ophthalmology. 1982;89:1357–61.
Shilling JS, Kohner EM. New vessel formation in retinal branch vein occlusion. Br J Ophthalmol. 1976;60:810–5. https://doi.org/10.1136/bjo.60.12.810.
Hayreh SS, Zimmerman MB. Fundus changes in branch retinal vein occlusion. Retina. 2015;35:1016–27.
Magargal LE, Brown GC, Augsburger JJ, Parrish RK 2nd. Neovascular glaucoma following central retinal vein obstruction. Ophthalmology. 1981;88:1095–101.
Zegarra H, Gutman FA, Conforto J. The natural course of central retinal vein occlusion. Ophthalmology. 1979;86:1931–9. https://doi.org/10.1016/s0161-6420(79)35327-1.
Hayreh SS, Rubenstein L, Podhajsky P. Argon laser scatter photocoagulation in treatment of branch retinal vein occlusion. Ophthalmologica. 1993;206:1–14. https://doi.org/10.1159/000310354.
Hayreh SS, Bridget Zimmerman M, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol.1994;117:429–41. https://doi.org/10.1016/s0002-9394(14)70001-7.
Chan CK, Ip MS, VanVeldhuisen PC, Oden NL, Scott IU, Tolentino MJ, et al. SCORE study report #11. Ophthalmology. 2011. https://doi.org/10.1016/j.ophtha.2010.11.020.
Mendis KR, Balaratnasingam C, Yu P, Barry CJ, McAllister IL, Cringle SJ, et al. Correlation of histologic and clinical images to determine the diagnostic value of fluorescein angiography for studying retinal capillary detail. Invest Ophthalmol Vis Sci. 2010;51:5864–9.
Spaide RF, Klancnik JM, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45. https://doi.org/10.1001/jamaophthalmol.2014.3616.
Nicholson L, Vazquez-Alfageme C, Patrao NV, Triantafyllopolou I, Bainbridge JW, Hykin PG, et al. Retinal nonperfusion in the posterior pole is associated with increased risk of neovascularization in central retinal vein occlusion. Am J Ophthalmol. 2017;182:118–25.
Acknowledgements
Presented at: the 19th EURETINA 2019 Congress, Paris, France, September 5-8, 2019. Acknowledgments to the NIHR Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, London, UK, for supporting the research.
Funding
HK is supported by a scholarship from Egyptian mission sector, Ministry of Higher Education, Egypt. RR has received speaker fees from Carl Zeiss Meditec AG. PAK has received speaker fees from Heidelberg Engineering, Topcon, Carl Zeiss Meditec AG, Haag-Streit, Allergan, Novartis, and Bayer. He has served on advisory boards for Novartis and Bayer and has been an external consultant for DeepMind and Optos. PAK is supported by a United Kingdom (UK) National Institute for Health Research (NIHR) Clinician Scientist Award (NIHR-CS--2014-12-023). Other authors have no financial disclosures. The sponsor had no rule in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huemer, J., Khalid, H., Wagner, S.K. et al. Phenotyping of retinal neovascularization in ischemic retinal vein occlusion using wide field OCT angiography. Eye 35, 2812–2819 (2021). https://doi.org/10.1038/s41433-020-01317-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01317-9
This article is cited by
-
Enhanced therapeutic effect of PEDF-loaded mesenchymal stem cell-derived small extracellular vesicles against oxygen-induced retinopathy through increased stability and penetrability of PEDF
Journal of Nanobiotechnology (2023)
-
Deep-learning-based AI for evaluating estimated nonperfusion areas requiring further examination in ultra-widefield fundus images
Scientific Reports (2022)