Learning Objectives
Upon completion of this activity, participants will be able to:
-
1.
Assess demographic risk factors for radiation retinopathy after I-125 plaque brachytherapy for uveal melanoma.
-
2.
Analyze foveal avascular zone size in comparing eyes among patients treated for uveal melanoma.
-
3.
Evaluate capillary density in comparing eyes among patients treated for uveal melanoma.
Continuing Medical Education
In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and Springer Nature. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s). Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at www.medscape.org/journal/eye; (4) view/print certificate.
Credit hours
1.0
Release date:
Expiration date: November 23rd 2021
Post-test link: https://medscape.org/eye/posttest938103
Authors/Editors disclosure information
S.S. has disclosed the following relevant financial relationships: Served as an advisor or consultant for: Allergan, Inc.; Apellis; Bayer AG; Boehringer Ingelheim Pharmaceuticals, Inc.; Heidelberg Pharma GmbH; Novartis; Oculis; Optos; Oxurion; Roche. Served as a speaker or a member of a speakers bureau for: Allergan, Inc.; Bayer AG; Novartis Pharmaceuticals Corporation; Optos. Received grants for clinical research from: Allergan, Inc.; Bayer AG; Boehringer Ingelheim Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; Optos. T.E.d.C. and W.F.M. have disclosed no relevant financial relationships.
Journal CME author disclosure information
Charles P. Vega, MD, has disclosed the following relevant financial relationships: Served as an advisor or consultant for: GlaxoSmithKline.
Abstract
Objectives
To determine if commercial OCTA measurements can provide quantitative biomarkers for detection of radiation retinopathy (RR) s/p I-125 plaque brachytherapy in patients with uveal melanoma.
Methods
Retrospective review of 6 × 6 mm OCTA images of nonirradiated fellow eyes (group 1, 28 eyes), eyes without RR (group 2, 22 eyes), eyes with RR (group 3, 13 eyes). We used automated AngioVue AngioAnalytics OCTA software determinations of FAZ size, perimeter size, and 27 capillary density measurements (nine regions of each segmentation: full-thickness retina, superficial plexus, deep plexus).
Results
Average time since irradiation was 1.9 years in group 2, and 3.7 years in group 3. FAZ size was 1.2 mm in group 3 compared with 0.2 mm in group 1 and 0.3 mm in group 2 (both p < 0.001). Capillary density was statistically significantly reduced in group 3 compared with group 1 in all 27 regions. Group 2 had significantly decreased superficial plexus capillary density compared with group 1 in three regions. Group 3 had significantly reduced capillary density compared with group 2 in 6/27 (22%) regions. Linear regression showed a change in whole-scan density of −1.5 per year after irradiation in the full-thickness retina segmentation (p = 0.008).
Conclusion
Quantitative OCTA may aid in early detection of RR.
Similar content being viewed by others
Introduction
Uveal melanoma is a life-threatening disease that is most commonly treated with I-125 plaque brachytherapy, which can damage the retina and choroid leading to radiation retinopathy and resulting vision loss [1]. Optical coherence tomography angiography (OCTA) has been utilized to study radiation retinopathy in a few studies in this patient population. In 2015, Veverka et al. showed that OCTA could detect retinopathy earlier than structural OCT or ophthalmoscopic findings, demonstrating its potential utility in evaluating radiation retinopathy [2].
Later studies attempted to quantify these changes. In 2016, Shields et al. published two papers in the journal Retina using Image J (National Institutes of Health, Bethesda, MD) and Adobe Photoshop CS3 (Adobe Systems Inc, San Jose, CA) to quantify 3 × 3 mm OCTA scans of the fovea and parafovea. The first paper showed a decrease in capillary density and foveal avascular zone (FAZ) size in eyes with radiation retinopathy compared with fellow eyes [3] The second paper demonstrated that eyes without radiation retinopathy that had previously undergone plaque brachytherapy may also have decreased capillary density [4].
However, in 2016 a novel software update was released by OptoVue called AngioVue AngioAnalytics (Optovue RTVue, XRAvanti, Optovue Inc., Fremont, CA) that allowed for automated quantification of OCTA scans including FAZ size, perimeter size, and capillary density measurements in each Early Treatment Diabetic Retinopathy Study (ETDRS) quadrant of the macula. This software was cleared by the FDA in 2018 and is now commercially available. This software update greatly standardized measurements and increased the ease of quantifying OCTA images without the need of exporting to and processing with outside software. To our knowledge the AngioVue AngioAnalytics software has only been used in one study of radiation retinopathy, which demonstrated decreased capillary density of the full-thickness inner retina of eyes before and 1 year after Ruthenium-106 plaque therapy [5]. While the results were interesting additions to the scientific literature, the study only skimmed the surface of what we can glean from the information the new software provides including FAZ measurements and capillary density measurements of each indivfidual retinal plexus. Furthermore, the study evaluated a different type of radiation (beta irradiation) in comparison to what is typically used in the United States of America (Iodine-125 low energy gamma irradiation).
The aim of the present study was to compare FAZ size, perimeter size, and 27 capillary density measurements (of the nine ETDRS grid regions of each segmentation: full-thickness inner retina, superficial plexus, deep plexus) of (1) fellow nonirradiated eyes, (2) eyes without radiation retinopathy from I-125 plaque brachytherapy for uveal melanoma, and (3) eyes with radiation retinopathy s/p I-125 plaque brachytherapy for uveal melanoma using the automated AngioVue AngioAnalytics software.
Subjects and methods
Patients
This study was approved by the Institutional Review Board of The University of Illinois at Chicago as an exemption from review due to its retrospective nature. The research adheres to the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act. This is a retrospective chart review of all patients with uveal melanoma who underwent I-125 plaque brachytherapy by a single surgeon (WFM) and who were imaged by the AngioVue AngioAnalytics software of the RTVue XR “Avanti” OCTA system by OptoVue (Optovue RTVue, XRAvanti, Optovue Inc., Fremont, CA). Presence of radiation retinopathy was determined by a single surgeon (WFM) based on presence of microvascular changes in the macula on ophthalmoscopy and/or presence of macular edema on structural OCT. Eyes with 6 × 6-mm macular OCTA scans at UIC between 1/1/2016 and 10/1/2018 were included. Eyes with other uveitic or vitreoretinal diseases, previous intraocular surgery except cataract surgery, and high myopia were excluded. Three OCTA images of poor quality defined as signal strength under two and/or image artifact obscuring at least ¼ of the image scan were also excluded. If a patient had multiple imaging sessions, only the most recent imaging date that met the above study protocol was included.
We separated the patients’ eyes into three groups: fellow nonirradiated control eyes (group 1), eyes s/p I-125 plaque brachytherapy without clinical radiation retinopathy (group 2), eyes s/p I-125 plaque brachytherapy with radiation retinopathy (group 3). The chart review included: date of imaging visit, patient age, patient gender, best corrected visual acuity (BCVA) at imaging visit, eye that underwent I-125 plaque brachytherapy, location of uveal melanoma, date of I-125 plaque brachytherapy, presence or absence of radiation retinopathy.
Optical coherence tomography angiography
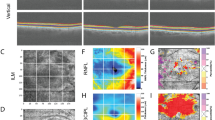
OCTA images were acquired by the AngioVue AngioAnalytics software of the RTVue XR “Avanti” OCTA system by OptoVue (Optovue RTVue, XRAvanti, Optovue Inc., Fremont, CA). The Avanti system operates at a scan rate of 70,000 a-scans per second with an axial resolution of 5 µm. The AngioVue AngioAnalytics software extracts a binary image from the OCTA image and then defines vessel density as the percentage of the area occupied by the microvasculature pixels in a given region. The vessel density is calculated for a variety of regions based on the ETDRS grid. The software automatically segments the retinal vasculature into full-thickness inner retina, superficial capillary plexus (SCP), and deep capillary plexus (DCP). The vessel density can therefore be calculated for each region within a specific OCTA segmentation.
The 6 × 6-mm OCTA macular scans were reviewed by a single grader (TED) to determine image quality and accuracy of automated retinal layer segmentation. As stated above, three OCTA images were excluded for poor image quality. The image signal strength was recorded for each eye. FAZ size and perimeter size measurements by the AngioAnalytics software were collected for each eye. Manual adjustment of the automated FAZ delineation was performed using the AngioVue AngioAnalytics software only when the automated FAZ delineation was deemed to be inaccurate by the grader. Capillary density measurements were recorded for each eye. These measurements included nine ETDRS regions (whole scan, temporal/superior/nasal/inferior parafovea, and temporal/superior/nasal/inferior perifovea) of each of the three segmentations (full-thickness inner retina, SCP, DCP) for a total of 27 regions analyzed per eye. In a few cases manual adjustment of the ETDRS grid was performed to center the grid at the fovea when the automated placement of the ETDRS grid was deemed to be inaccurate by the grader. In these cases, the capillary density numbers were automatically recalculated by the AngioVue AngioAnalytics software after adjustment of the ETDRS grid. If the image quality was poor in a particular region but the entire OCTA image otherwise met criteria for entrance into the study, only that particular region was removed from the study. Only 63/1701 (3.7%) regions were removed from analysis for these purposes.
Statistical analysis
XLSTAT of Microsoft excel (Office 365 ProPlus, version 1808 build 10730, 2018, Redmond, Washington) was used for all statistical analysis and p values of <0.05 were considered statistically significant. The Shapiro–Wilk test was used to determine that the data sets had a non-normative distribution. A Kruskal–Wallis test for non-normative distribution was therefore used to compare the average age, BCVA, OCTA signal strength, and all OCTA measurements between groups 1, 2, and 3. If a statistical significance was noted between the three groups for any factor, a Dunn’s multiple comparisons test with bonferroni correction (p < 0.017 significance value) was performed to determine if there was any difference between groups 1 and 2, groups 2 and 3, and groups 1 and 3. A Chi-squared test was used to compare the proportion of females and proportion of right eyes between groups 1, 2, and 3. A Chi-squared test was also used to compare the proportion of eyes with peripapillary or macula uveal melanoma versus peripheral uveal melanoma between groups 2 and 3. A Mann–Whitney comparison of two samples was used to compare the number of years since I-125 plaque brachytherapy between groups 2 and 3. A linear regression was used to plot FAZ size, perimeter size, whole-scan capillary density measurements for each segmentation (full-thickness retina, SCP, and DCP), and LogMAR BCVA over time since I-125 plaque brachytherapy in all irradiated eyes (groups 2 and 3 combined) to determine the change in these measurements over time.
Results
A total of 63 eyes were included in this study: 28 eyes in group 1 fellow nonirradiated eyes, 22 eyes in group 2 without radiation retinopathy s/p I-125 plaque brachytherapy, and 13 eyes in group 3 with radiation retinopathy s/p I-125 plaque brachytherapy.
In group 1 there were 16 females (57%) and 20 right eyes (71%). The average age was 59 (range 43–82) years; the average BCVA was 0.06 LogMAR (range 0–0.3); the average OCTA signal strength was 7.5 (range 5–9).
In group 2 there were 6 females (27%) and 7 right eyes (32%). The average age was 58 (range 26–76) years; the average BCVA was 0.16 LogMAR (range 0–0.4); the average OCTA signal strength was 6.7 (range 3–9). The uveal melanoma was in the macula and/or at the arcade in four eyes (18%), peripapillary in one eye (5%), and in the periphery in the other 17 eyes (77%). The average years since irradiation was 1.9 (range 0.3–6) years.
In group 3 there were nine females (69%) and eight right eyes (62%). The average age was 63 (range 47–82) years; the average BCVA was 0.78 LogMAR (range 0.2–1.7); the average OCTA signal strength was 5.5 (range 2–10). The uveal melanoma was in the macula and/or at the arcade in five eyes (38%), peripapillary in two eye (15%), and in the periphery in the other six eyes (46%). The average years since irradiation was 3.7 (range 1.2–7.2) years.
There was no statistically significant difference between the three groups in terms of age (p = 0.67), sex (p = 0.05), or eye involved (p = 0.17). There was a significant difference in LogMAR BCVA between all three groups: group 1 < 2 (p = 0.001), group 1 < 3 (p = <0.001), group 2 < 3 (p = 0.003). Linear regression showed a worsening of BCVA of +0.09 LogMAR per year (a decrease of approximately one line of Snellen visual acuity per year, p = 0.02). While there was not a significant difference in signal quality between groups 1 and 2 (p = 0.28) and groups 2 and 3 (p = 0.12), there was a decrease in signal quality in group 3 compared with group 1 (p = 0.01). It was more common for the uveal melanoma to be macular or peripapillary in group 3 (43%) than in group 2 (23%) but this comparison did not reach statistical significance (p = 0.06).
Size of the FAZ was 1.2 mm in group 3 compared with 0.2 mm in group 1 and 0.3 mm in group 2 (both p < 0.001, Table 1). Linear regression showed an increase in the FAZ size of +0.087 mm per year (p = 0.07) after the one outlier greater than two standard deviations away was removed (Fig. 1). Perimeter size was also significantly different between groups 1 and 3 and groups 2 and 3 (both p < 0.001, Table 1). Linear regression showed an increase in perimeter size of +0.35 mm per year (p = 0.12) after the one outlier greater than two standard deviations away was removed.
The average capillary density measurements in the 27 ETDRS regions for each group are listed in Table 2 and the statistical comparisons of these capillary density between the three groups are noted in Table 1. Capillary density was statistically significantly reduced in group 3 compared with group 1 in all 27 regions (ranging p = 0.011 to p < 0.001). Group 2 had a statistically significant decrease in capillary density in 3/27 regions (11%) compared with group 1, all of which were regions of the SCP. Finally, group 3 had statistically significantly reduced capillary density compared with group 2 in 6/27 (22%) ETDRS grid regions. Linear regression showed a decrease in whole OCTA scan capillary density of −1.5 per year in the full-thickness retina segmentation (p = 0.008, Fig. 2), −1.5 per year in the SCP segmentation (p = 0.008), and −1.4 per year in the DCP segmentation (p = 0.01).
Discussion
This manuscript is the first to compare automated OCTA measurements between eyes with uveal melanoma s/p I-125 plaque brachytherapy with and without retinopathy and fellow nonirradiated eyes. Most previous studies used proprietary or external software to show a difference in capillary density after irradiation. However, in our study we utilized the commercially available automated AngioVue AngioAnalytics software, making our study more directly clinically pertinent. Further we opted to compare not just eyes with retinopathy to control eyes but also looked at the subset of patients without clinical retinopathy to determine if OCTA could detect retinal vascular changes earlier than clinical examination alone.
First, this study confirmed the results of prior studies by demonstrating that eyes with radiation retinopathy (group 3) had a statistically significant decrease in capillary density and increase in FAZ size and perimeter size than fellow nonirradiated eyes (group 1). The decrease in capillary density was noted in all retinal segmentations. Second, we showed that the SCP capillary density measurements may be useful in detecting early radiation retinopathy as there appeared to be a decrease in the capillary density in this capillary plexus of irradiated eyes without clinical retinopathy (group 2) compared with nonirradiated eyes. We suspect that groups 2 and 3 are both parts of the spectrum of radiation retinopathy but that signs of retinopathy present in group 2 are not readily detectable with previous methods of evaluation. Third, FAZ size was enlarged between groups 1, 2, and 3 showing that this could also be a good early indicator of retinopathy. Early detection of FAZ size increase or capillary density changes in the SCP may be useful biomarkers that prompt clinicians to monitor patients more carefully possibly leading to earlier treatment of the sequelae.
Many other retinal vascular diseases also demonstrate progressive FAZ enlargement and decreased capillary density measurements [6,7,8]. However, it is interesting to note that in most retinal vascular diseases, such as retinal vein occlusions or diabetic retinopathy, the DCP and SCP are thought to be disproportionately affected, and many people believe that the DCP may be affected to a greater degree or earlier on than the SCP [9,10,11]. This is in contrast to the findings in the present study, which demonstrated a decreased capillary density in regions of the SCP in eyes without clinical retinopathy but no significant decrease in the DCP. This suggests that the SCP may actually be affected earlier than the DCP in eyes s/p I-125 plaque brachytherapy. Further studies with larger sample sizes may better elucidate if the SCP is truly affected earlier than the DCP as our study suggests especially as a handful of the SCP capillary density measurement comparisons between group 1 and 2 were only minimally above the Bonferroni corrected p value of 0.017.
In this study we also used the data from all eyes that were s/p I-125 plaque brachytherapy (combining groups 2 and 3) to perform linear regressions to predict the change over time of BCVA (a decrease in nearly one line of Snellen visual acuity per year) and OCTA measurements. The decrease in vision over time is interesting to note as even eyes without clinical retinopathy demonstrated some visual acuity decrease over time. This decrease in visual acuity may be secondary to cataract but could also suggest that even without macular edema there may be a component of damage such as non-perfusion that is limiting these patients’ vision. Linear regression also showed that the FAZ increased by +0.087 mm per year and that the capillary density decreased by −1.5 per year in the full-thickness retina and SCP segmentations and by −1.4 per year in the DCP. These linear regressions may provide clinicians and our patients with the ability to better predict how much damage may occur over time. With better understanding of the damage over time, we may adjust our follow-up time frame accordingly for patients at high risk of potential vision loss from radiation retinopathy. It would be beneficial to repeat these calculations with a larger sample size including patients that have longer follow-up times to confirm these calculations. With larger sample sizes it would also be possible to separate patients into high-risk and low-risk groups for developing radiation retinopathy. For example, eyes with higher doses of radiation and radiation closer to the fovea or optic nerve are likely to progress faster and therefore may have a steeper trajectory than the overall cohort. While our study did not have the power to definitively show that eyes with I-125 plaque brachytherapy closer to the fovea and optic nerve were more likely to develop retinopathy, there was a trend toward this notion and prior literature has already demonstrated that radiation retinopathy is more likely to occur in posteriorly located uveal melanoma cases than with more peripheral lesions [12,13,14]. Location of the melanoma does not directly affect image acquisition. However, presence of macular edema, which is more common in posterior pole melanomas, can decrease signal quality to the deeper layers of the OCTA.
The main weaknesses of the paper include the relatively modest sample size, retrospective nature, and the difference in signal strength between groups 1 and 3. The average signal strength was only 5.5 in group 3 compared with 6.7 in group 2 (p = 0.12) and 7.5 in group 1 (p = 0.01). A decrease in signal strength can affect capillary density measurements by reducing contrast between flow (retinal vasculature) and non-flow areas, and by creating artifact and/or image noise. This can produce falsely high capillary density measurements. Previous studies have shown that image quality can affect the reproducibility of capillary density measurements [15]. In our study, three eyes with poor quality OCTA images were removed to minimize this issue. However, it is possible that the capillary density measurements in group 3, and to some degree in group 2, may have been falsely high due to the lower signal quality. Therefore, there may actually be an even larger difference between group 1 and groups 2 and 3 than noted in this study. While the overall conclusions of the study remain unchanged by this, future prospective studies would be useful to ensure more even and consistent signal strength values. While the most clinically relevant area is the posterior pole, which we evaluated in this study, future studies could employ wide-field OCTA or a montage technique for further information.
In conclusion, the present study demonstrated that automated FAZ size and capillary density measurements may be useful biomarkers for early detection of radiation retinopathy after I-125 plaque brachytherapy, and determined the change in these measurements each year using linear regression. These findings provide us with a greater understanding of the disease process and a way to use commercially available imaging to monitor our patients for radiation retinopathy more closely.
Summary
What was known before
-
Prior studies used proprietary software to note that OCTA shows a decrease in capillary density and increase in FAZ size in eyes with radiation retinopathy compared with fellow eyes [3]. Eyes without radiation retinopathy that had previously undergone plaque brachytherapy may also have decreased capillary density [4]. One study used the new commercially available automated OCTA software to show decreased capillary density of the full-thickness inner retina of eyes before and 1 year after Ruthenium-106 plaque therapy [5].
What this study adds
-
Our study compared FAZ size, perimeter size, and 27 capillary density measurements (of the nine ETDRS grid regions of each segmentation: full-thickness inner retina, superficial plexus, deep plexus) of (1) fellow nonirradiated eyes, (2) eyes without radiation retinopathy from I-125 plaque brachytherapy for uveal melanoma, and (3) eyes with radiation retinopathy s/p I-125 plaque brachytherapy for uveal melanoma using the automated AngioVue AngioAnalytics software. Therefore, our study is different because it used commercially available software, looked at all three groups in tandem, looked at many more segmentations and area measurements, and performed linear regressions to show change over time of these potential biomarkers. It is thus more clinically applicable and could potentially be used in the future for prognostication and monitoring.
References
Shields CL, Shields JA. Recent developments in the management of choroidal melanoma. Curr Opin Ophthalmol. 2004;15:244–51.
Veverka KK, Abouchehade JE, Iexxi R, Pulido JS. Noninvasive grading of radiation retinopathy—the use of optical coherence tomography angiography. Retina. 2015;35:2400–10.
Shields CL, Say EAT, Samara WA, Khoo CTL, Mashayekhi A, Shields JA. Optical coherence tomography angiography of the macula after plaque radiotherapy of choroidal melanoma—comparison of irradiated versus nonirradiated eyes in 65 patients. Retina. 2016;36:1493–505.
Say EAT, Samara WE, Khoo CTL, Magrath GN, Sharma P, Ferenczy S, et al. Parafoveal capillary density after plaque radiotherapy for choroidal melanoma—analysis of eyes without radiation maculopathy. Retina. 2016;36:1670–8.
Cennamo G, Breve MA, Velottia N. Evaluation of vascular changes with optical coherence tomography angiography after plaque radiotherapy of choroidal melanoma. Ophthalmic Res. 2018;60:238–42.
de Carlo TE, Chin AT, Bonini Filho MA, Adhi M, Branchini L, Salz DA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35:2364–70.
Nesper PL, Roberts PK, Onishi AC, Chai H, Liu L, Jampol LM, et al. Quantifying microvascular abnormalities with increasing severity of diabetic reinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58:BIO307–15.
Salz DA, de Carlo TE, Adhi M, Moult E, Choi W, Baumal CR, et al. Select features of diabetic retinopathy on swept source optical coherence tomography angiography compared with fluorescein angiography and normal eyes. JAMA Ophthalmol. 2016;134:644–50.
Adhi M, Filho M, Louzada R, Kuehlewein L, de Carlo TE, Baumal CR, et al. Retinal capillary network and foveal avascular zone in eyes with vein occlusion and fellow eyes analyzed with optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:OCT486–94.
Onishi AC, Nesper PL, Roberts PK, Moharram GA, Chai H, Liu L, et al. Importance of considering the middle capillary plexus on OCT angiography in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:2167–76.
Simonett JM, Scarinci F, Picconi F, Giorno P, De Geronimo D, Di Renzo A, et al. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes. Acta Ophthalmol. 2017;95:e751–5.
Aziz HA, Singh N, Bena J, Wilkinson A, Singh AD. Vision loss following episcleral brachytherapy for uveal melanoma: development of a vision prognostic tool. JAMA Ophthalmol. 2016;134:615–20.
Gunduz K, Shields CL, Shields JA, Cater J, Freire JE, Brady LW. Radiation retinopathy following plaque radiotherapy for posterio uveal melanoma. Arch Ophtalmol. 1999;117:609–14.
Krema H, Xu W, Vasquez LM, Pavlin CJ, Simpson R. Factors predictive of radiation retinopathy post (125)Iodine brachytherapy for choroidal melanoma. Can J Ophthalmol. 2011;46:158–63.
Fenner BJ, Tan GSW, Tan ACS, Yeo IYS, Wong TY, Cheung GCM. Identification of imaging features that determine quality and repeatability of retinal capillary plexus density measurements in OCT angiography. Br J Ophthalmol. 2018;102:509–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
de Carlo, T.E., Mieler, W.F. Automated quantitative OCTA measurements of uveal melanoma-treated eyes with and without radiation retinopathy after I-125 plaque therapy and of nonirradiated fellow eyes. Eye 35, 769–776 (2021). https://doi.org/10.1038/s41433-020-01237-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01237-8
This article is cited by
-
Radiation Retinopathy
Current Ophthalmology Reports (2023)