Abstract
Objective
To compare fibrin glue (with three cardinal sutures) (FG) and polygalactin suture (PS) for mucous membrane grafting (MMG) in terms of graft apposition and recurrence of lid margin keratinization (LMK) and metaplastic lashes (ML) in patients with Stevens–Johnson syndrome (SJS).
Design
Prospective randomized comparative interventional study.
Methods
Twenty patients diagnosed with SJS and lid margin abnormalities including LMK with or without ML were randomized to undergo either fibrin glue (FG)-assisted MMG (n = 10) or continuous 8-0 polygalactin suture (PS)-assisted MMG (n = 10). They were evaluated preoperatively and during follow-up at 1 week and 1, 2, 3, and 6 months. The parameters assessed were best-corrected visual acuity (BCVA), tear break-up time (TBUT), Schirmer-1 test, corneal and conjunctival complications, graft apposition and width (GW), LMK, ML, impression cytology, and operative time. The primary outcome measures are incidence of graft displacement and recurrence of LMK and ML.
Results
None of the eyelids in FG group (0/40) and 1 eyelid in PS group (1/40) had graft displacement. Recurrence of LMK occurred in 7.5% of eyelids (3/40) in both the study groups. Recurrence of ML occurred in 2.5% (1/40) in FG group and 5% (2/40) in PS group. The mean operative time for MMG in FG group was 39.5 ± 2.40 min and in PS group was 56 ± 1.63 min (p = 0.001).
Conclusions
As graft apposition with suture involves significantly longer intraoperative time, if cost is not a limiting factor then fibrin glue is a viable option for the MMG for lid margin pathologies.
Similar content being viewed by others
Introduction
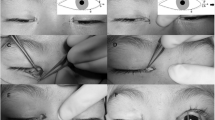
Stevens–Johnson syndrome (SJS) is an immune-mediated disease complex involving skin and mucosal surfaces and is associated with a spectrum of ocular manifestations. It is characterized by an acute stage of inflammation and epithelial erosions and the chronic stage of cicatrization. Scarring in the chronic stage involves the tarsus, conjunctiva, and the cornea and may lead to lid margin pathologies such as keratinization (LMK), metaplastic lashes (ML), and entropion (Fig. 1). These lid pathologies can further result in chronic ocular surface irritation and inflammation and subsequent blindness [1]. The utility of large diameter contact lenses has been studied in these eyes to reduce the blink-related microtrauma and discomfort but poor patient compliance, and associated complications are the major limitations for this treatment approach [2]. A definitive solution is to surgically correct the lid margin pathologies i.e., to replace the keratinized conjunctiva or augment the deficient conjunctiva with a mucosal graft. There are reports of mucous membrane grafting (MMG) for LMK in patients with SJS and other ocular cicatricial conditions to reduce the friction on the ocular surface. These studies showed that the procedure helps in the resolution of the ocular surface inflammation and improves the eyelid closure, thereby improving the ocular surface [3, 4].
Fibrin glue (FG) has been used instead of suture fixation in surgeries for pterygium excision and conjunctival autografting and ocular surface reconstruction for the apposition of conjunctiva and amniotic membrane graft (AMG), respectively [5,6,7]. Multiple comparative studies on using FG and suture fixation have shown that the use of FG is associated with significantly less postoperative pain and discomfort and also shortens the operative time [8,9,10]. None of these studies have shown any adverse reactions, inflammation, or delay in healing although graft displacement has been reported in few patients [8,9,10]. Further, these studies demonstrated that the pterygium recurrence rate was similar with both the grafting procedures. The mucous membrane graft being a thicker graft than the conjunctival autograft and AMG, when apposed to the lid margin with FG may get displaced due to constant rubbing of the lid margin against the ocular surface during blinking. Hence, we conducted a prospective randomized study with the primary aim to compare the apposition of mucous membrane graft to the lid margin with FG with 3 cardinal sutures (FG) and continuous 8-0 polygalactin suture (PS) in patients with LMK with or without ML.
Methods
This was a prospective, randomized, comparative, interventional study conducted at a tertiary eye care center. Approval from the institute ethics committee was taken prior to the commencement of the study and the study complies with the tenets of the Declaration of Helsinki. A written informed consent was obtained from all patients prior to the inclusion into the study.
Twenty patients (40 eyes) with SJS were recruited from the Oculoplasty and Cornea clinics and were randomized into the two study groups based on a randomization sequence generated using randomly permuted blocks and a randomization table created for the 20 patients, with 1:1 treatment allocation. Patients underwent excision of LMK and MMG with either FG (n = 10) or PS (n = 10).
Patients with SJS aged more than 10 years with lid margin abnormalities (LMK with or without ML) were included in the study. The diagnosis of SJS was based on a confirmed history of acute onset of high fever, serious mucocutaneous illness with skin eruptions, and involvement of at least two mucosal sites including the ocular surface. Patients with extensive symblepharon (grade 2, 3), where fornix reconstruction with posterior lamellar augmentation was also required and those with lagophthalmos of more than 2 mm or in whom entropion correction was needed, were excluded. After the recruitment, a detailed preoperative workup was done.
Pertinent clinical history including demographic information, etiological factors (drug allergy/fever), duration of initial insult to time of presentation, history of previous surgeries, history of any ocular symptoms was noted. Preoperative best-corrected visual acuity (BCVA) was measured using Snellen chart. The severity of dry eye was assessed in terms of tear break-up time (TBUT) and Schirmer-1 test. The baseline conjunctival hyperemia and staining, corneal staining characteristics, epithelial defect, neovascularization, conjunctivalization, opacification and keratinization were graded as per the new simplified grading system given by Sotozono et al. [11].
The extent of keratinized lid margin was examined, and the total area of the upper and lower eyelids with keratinization was scored as 0–3, where grade 0 is no LMK, grade 1 is keratinization involving less than one-third of the lid margin, grade 2 is keratinization involving one-third to two-thirds of the lid margin, and grade 3 is keratinization involving more than two-thirds of the lid margin [11]. Furthermore, the lid margin was examined, and the presence of ML (arising from the meibomian gland orifices), trichiasis, and entropion was documented.
The specimens for impression cytology were obtained from the temporal and nasal cornea and bulbar conjunctiva with the intervening limbus using a 0.22-micron millipore cellulose acetate filter paper. The slides were prepared and stained with haematoxylin and eosin. Ten high power fields were examined for goblet cells and epithelial cells. The specimens were taken preoperatively and at 3 months postoperative follow-up visit.
Surgical technique
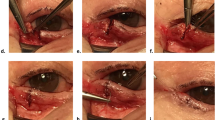
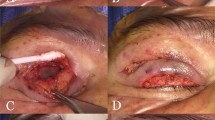
The surgery was done by single surgeon (NP) under local/general anesthesia (Fig. 2). The surgical sites were cleaned (with povidone-iodine 10%) and draped. Traction sutures were placed at the upper and lower lid margin to evert the lid over a spatula so as to reveal the lid margin and the tarsus, and the keratinized conjunctiva was excised. In patients with ML, incision was given at the gray line, and strip of posterior lamella along with the roots of ML was excised. The horizontal and the vertical dimensions of the defect was measured using a calliper. All the affected eyelids were operated in single setting. Then, a strip of full thickness mucosal graft, a little larger than the defect was harvested from the labial mucosa after submucosal injection of 2% lignocaine with 1:100,000 epinephrine. The donor site was searched for the bleeding points, which were cauterized. The excess fat was cleared from the stromal surface of the graft. The graft was placed over the defect in the lid margin. In the FG group, three cardinal sutures (8-0 polygalactin) were placed each at the center, medial, and lateral ends of the graft, and FG (Tisseel VH fibrin sealant; Baxter AG, Vienna, Austria) was applied to secure the graft on the lid margin in the FG group. In the PS group, continuous interlocking 8-0 PS was used to secure the graft along the lid margin, and simple continuous suturing was used along the conjunctival edge. Antibiotic ointment was applied, and the operated eye was bandaged for 24 h. The time taken for each surgery was documented.
The photograph shows the sequential steps of mucous membrane grafting using fibrin glue. a–c Cardinal sutures were placed each at the center, medial, and lateral ends of the graft. d Fibrin glue was applied to secure the graft on the lid margin. e, f The graft is pressed firmly against the lid margin to ensure it strongly adheres to it.
After surgery, all patients received topical antibiotics, steroids, and artificial tears. Lidocaine hydrochloride oral topical solution 2% was prescribed to be applied at the donor site before meals, povidoneiodine 1% mouth gargles after every meal. Topical antibiotics and steroids were tapered over a period of 6 weeks, and lubricants were continued.
Postoperatively, the clinical parameters were assessed at 1, 4 weeks and 2, 3, and 6 months. Patients were asked to report the percentage improvement they felt at the 3 months’ postoperative period compared to the baseline.
Outcome measures
The primary outcome measures were the percentage of eyelids with incidence of graft displacement and the recurrence of LMK and ML. The secondary outcome measures were the average graft width, change in BCVA, dry eye parameters, corneal and conjunctival complications, ocular surface improvement documented by impression cytology, percentage subjective improvement (experienced by the patient), and operative time. The width of the graft was measured at the center, medial 1/3rd and lateral 1/3rd of the lid margin for each eyelid in millimeters through slit-lamp micrometer by single observer for all patients at all postoperative examinations, and the average graft width was documented.
Data were analyzed using statistical software Stata 14. Qualitative data were expressed as frequency and percentage and quantitative data were expressed as mean ± SD and median (minimum–maximum) when following normal and skewed distribution, respectively. Normality of the data was checked by using the Shapiro–Wilk test. Repeated measure ANOVA test followed by Bon Ferroni correction was used to check change over a period of time for normally distributed data. Friedman test followed by the Wilcoxon signed-rank test was used to compare change over a period of time for skewed data. Categorical data were compared by using the Chi-square/Fisher-exact test. Independent t-test and rank sum test were used to compare continuous variables between the groups. A p < 0.05 is considered to be significant.
Results
A total of 20 patients were recruited during the study period (September, 2016 and October, 2017) and were randomized into two study groups, 10 patients in the FG group and 10 patients in the PS group. Baseline parameters such as age, gender distribution, BCVA, TBUT, and Schirmer-1 test scores were comparable between the study groups. The mean age was 20.50 ± 3.72 years (mean ± SD) in the FG group and 23.60 ± 10.82 years (mean ± SD) in the PS group (p = 0.39). Male to female subjects’ ratio was 1:1 (n = 10) in each study group. The median duration of the illness was 122 months (range, 36–264 months) in the FG group and 122 months (range, 36–180 months) in the PS group (p = 0.604). The etiology of SJS was drug allergy in 70% of the patients (n = 10) in the FG group and in 90% (n = 10) in the PS group. Fever was the inciting factor in rest of the patients. Seventy-five percent (15/20) of eyes in the FG group and 75% (15/20) of the eyes in the PS group had grade-3 LMK (p = 0.58) (Fig. 1). ML were present in 32.5% (13/40) of eyelids in the FG group and 45% (18/40) of eyelids in the PS group (p = 0.384). The preoperative clinical parameters are mentioned in Table 1.
There was no incidence of graft displacement in the FG group (Figs. 3 and 4) whereas one eyelid had graft displacement over lateral one-third of the lid margin due to broken suture in the PS group. The displaced graft was cut and removed, and the rest of the apposed graft was left intact and there was no recurrence of LMK till 6 months follow-up period.
Out of 40 eyelids operated in each group, recurrence of keratinization occurred in 3 eyelids (7.5%, 3/40) at 3 months’ postoperative period in both the groups (FG and PS). The recurrence occurred at the edges and did not involve the mucous membrane graft. Furthermore, one eyelid (2.5%, 1/40) in the FG group and two eyelids (5%, 2/40) in the PS group developed recurrent metaplastic eyelashes.
The mean graft width showed statistically significant decline over the follow-up visits in both the study groups (p = < 0.001). The magnitude of graft shrinkage was statistically comparable between the FG and PS groups at each follow-up (Table 2). The graft width could not be measured at 6 months’ postoperative period as the edges of the mucous membrane graft merged with the adjacent conjunctiva, and it was difficult to delineate the margin of the graft.
The BCVA showed significant improvement from preoperative period to the postoperative 6 months in both the study groups. The median BCVA in FG and PS groups in logMAR units was 0.385 (0–2.48, range) and 1.78 (0–2.48, range) at baseline (p = 0.06), 0.30 (0–2.48) and 0.39 (0–2.48) at 6 months (p = 0.05), respectively. The p value for intragroup comparison between BCVA at preoperative and postoperative 6 months was 0.008 in FG group and 0.008 in PS group. The p values for the intergroup comparison at each follow-up time point were statistically non-significant.
The dry eye parameters (TBUT and Schirmer-1 test scores) showed statistically significant improvement following MMG in both the study groups with no significant intergroup difference (Table 3). Most of the eyes in both the study groups showed improvement in the conjunctival hyperemia, staining scores, and corneal staining scores but statistically significant improvement was not seen in other corneal parameters (Table 4). The corneal staining scores showed significant improvement from the preoperative period to the 6 months follow-up in both the study groups (p = 0.002 in FG group and p = 0.001 in PS group). Sixteen eyes (80%) in the FG group and 17 eyes (85%) in the PS group showed improvement in the corneal staining scores at 6 months follow-up. There was statistically significant difference between the study groups at postoperative 4 weeks (p = 0.045), 2 months (p = 0.014), and 6 months (p = 0.026). The improvement was seen earlier in the FG group compared to PS group.
The mean operative time (per patient) for MMG in FG group was 39.5 ± 2.40 min and in PS group was 56 ± 1.63 min (p = 0.001). The median subjective percentage improvement assessed by the patient from the preoperative period to the postoperative 3 months was 90% (range, 60–95%) in FG group and 90% (range, 80%-95%) in PS group (p = 0.09). The results of cytological analysis are shown in Table 5.
Discussion
MMG has been shown to be an effective surgical approach in the correction of LMK in patients with SJS [3, 4]. In the present study, 7% (3/40) of eyelids in FG group and 7% (3/40) in PS group developed signs of recurrent LMK and rest of the lid margin grafts remained free of keratinization till 6 months follow-up in both the groups (Fig. 5). In eyelids with ML, we removed full thickness posterior lamellae including the segment with ML, which could be the reason behind the low recurrence of ML observed in such cases.
Literature search revealed only one retrospective case series of MMG for the lid margin pathologies employing FG [3]. The authors excised the keratinized lid margin to a width of 5 mm and apposed the outer or anterior edge of the mucosal graft using 8-0 vicryl continuous suture and the inner edge was apposed to the raw tarsal surface with the FG (Baxter, Tisseel Kit). In the present study, we have used only three cardinal sutures to anchor the graft instead of suturing the entire anterior edge before apposing it with the FG. This is to ensure that the graft apposition is done in toto with the FG with minimal use of sutures so as to ensure homogeneity in the comparison of the two grafting techniques (FG vs. PS).
The role of FG as a tissue adhesive has been studied extensively in the ophthalmic literature. Several studies comparing conjunctival autografting with FG with that of conventional suturing technique following pterygium excision have found that the use of FG resulted in decreased postoperative pain and shorter operating time [8,9,10]. In the present study, we observed that the operative time was significantly reduced with the application of FG for apposition of the mucous membrane grafts when compared to suturing (p = 0.001). Shorter operative time may reduce the surgical costs, patient discomfort (in cases operated under local anesthesia), and the risks of general anesthesia.
An earlier report has hypothesized that the application of FG for attaching the mucous membrane graft eliminates the need for suturing along the inner edge, which itself can cause a considerable amount of surface irritation [3]. In the present study, the ocular surface of eyes, operated with suture assisted MMG, assessed in terms of conjunctival hyperemia and staining scores, were similar to those operated with FG fixation. However, the improvement in the corneal staining characteristics occurred earlier in the FG group when compared to that in the PS group even though there was no difference observed at the 6 months’ postoperative period. This observation shows that the presence of sutures delayed the improvement of ocular surface proving that the PS employed for the graft apposition may cause additional ocular surface irritation. The marked improvement observed in the conjunctival hyperemia and staining characteristics is in accordance with the previous studies on MMG for lid margin pathologies, which reported that conjunctival inflammation improves significantly following the removal of the keratinized lid margin and its replacement with the mucosal graft [3, 4].
The single event of graft displacement in our study occurred in the PS group. The lateral 1/3rd of the graft was displaced since the suture knot was opened up at that site. The displaced graft was removed, and the rest of the graft remained intact. There was no incidence of graft displacement in the FG group. Thus, the mucous membrane grafts apposed to the lid margin with the FG (along with three cardinal sutures) do not have a higher risk of graft displacement compared to the grafts sutured to the lid margin. This observation supports the results of the previous studies on the comparison of FG and suture fixation in conjunctival autografting in the pterygium surgery [8,9,10]. The magnitude of graft shrinkage was statistically comparable between the FG and PS groups; however, there was significant shrinkage of MMG in both the groups because of secondary contraction. Furthermore, at 6 months follow-up it was difficult to delineate the margins of the MMG as it merges with the conjunctiva.
Dry eye is a significant chronic ocular complication of SJS and an important cause of ocular discomfort in these patients [12]. In the present study, the schirmer-1 test scores and the tear film stability (measured as TBUT) showed statistically significant improvement following the MMG in both the study groups. Iyer et al. hypothesized that the goblet cells in the lip mucosa may have contributed to the mucin over the ocular surface leading to the better tear film stability [3]. However, since the transplanted oral mucosal grafts do not contain goblet cells [13, 14], and the submucosal gland density in the harvested oral mucosa grafts is low (due to intentional removal to ensure thin uniform grafts), the authors understand that it is rather the absence of LMK and the relief from the chronic blink-related microtrauma that has improved the ocular surface and contributed to the better tear film stability than the mucin produced from the remnant goblet cells from the mucosal grafts. It is interesting to note that the nasal mucosal grafts have higher density of goblet cells [13]; however, their feasibility as a lid margin graft is yet to be studied.
The changes in ocular surface have been assessed through impression cytology (Fig. 6). Impression cytology helps assess the limbal stem cell deficiency (LSCD), which is an important causative factor for the chronic corneal complications seen in patients with SJS. Araujo et al. correlated corneal changes seen on in vivo confocal microscopy with cytological findings in patients with total LSCD, wherein the common findings in both the investigations were squamous metaplasia, inflammatory cell infiltration, goblet cell depletion, and nuclear snake-like chromatin pattern [15]. In our series, the predominant cytological feature observed was acute inflammatory cells seen in both the groups (7/20, 35% in FG group and 6/20, 30% in PS group), which resolved after the lid margin MMG. It has been suggested in an earlier report that the lid margin MMG reduces the chronic inflammation and the blink-related ocular surface trauma [3]. The present study provides a cytological evidence to support this suggestion. The keratinization of the corneal surface was another common cytological feature observed on impression cytology. The keratinized corneal epithelium is an evidence of the squamous metaplasia of the ocular surface as a response to the chronic mechanical and inflammatory insult. Fatima et al. reported that the absence of goblet cells on the corneal specimens does not rule out the LSCD, and squamous metaplasia is a significant indicator of chronic ocular surface damage [16]. In our series, goblet cells were seen in very few patients and some had features of keratinized corneal epithelium, where all the patients had clinical features of LSCD. The keratinization did not resolve after the lid margin MMG since it is a chronic metaplastic change and may not reverse after the resolution of inflammation or require longer time to show any change.
The smaller sample size is an important limitation of the present study and further research with larger sample size and longer follow-up duration may help identify the rarer complications and long-term efficacy of the MMG. In conclusion, MMG for lid margin pathologies is a successful surgery in terms of clinical outcomes and patient satisfaction. FG is as effective as PS for graft apposition, with similar recurrence of LMK and ML and the magnitude of shrinkage of the graft. Although, presence of PS caused delay in the corneal surface healing, at the end of 6 months, both the graft apposition techniques (FG and PS) resulted in similar ocular surface improvement. As the graft apposition with continuous sutures involves significantly longer intraoperative time and delay in corneal surface healing, FG is a viable option for the apposition of mucous membrane graft in the correction of lid margin pathologies with similar clinical outcomes.
Summary
What was known before
-
MMG for lid margin pathologies in patients with SJS effectively improves the ocular surface health.
-
The two graft apposition techniques described were suturing the graft to the lid margin and attaching the graft with FG (after suturing the outer edge of the graft).
What this study adds
-
A novel surgical technique of apposing the mucous membrane graft to the lid margin with FG and just three cardinal sutures has been described.
-
The present study has proven that the incidence of graft displacement in the mucous membrane grafts attached to the lid margin with FG is similar to that observed in the grafts sutured to the lid margin.
References
Dipascuale M, Espana E, Liu D, Kawakita T, Li W, Gao Y, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens–Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology. 2005;112:904–12.
Pullum K, Buckley R. Therapeutic and ocular surface indications for scleral contact lenses. Ocul Surf. 2007;5:40–8.
Iyer G, Pillai VS, Srinivasan B, Guruswami S, Padmanabhan P. Mucous membrane grafting for lid margin keratinization in Stevens–Johnson syndrome: results. Cornea. 2010;29:146–51.
Fu Y, Liu J, Tseng SCG. Oral mucosal graft to correct lid margin pathologic features in cicatricial ocular surface diseases. Am J Ophthalmol. 2011;152:600–608.e1.
Koranyi G, Seregard S, Kopp ED. Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol. 2004;88:911–4.
Sharma N, Thenarasun SA, Kaur M, Pushker N, Khanna N, Agarwal T, et al. Adjuvant role of amniotic membrane transplantation in acute ocular stevens-johnson syndrome: a randomized control trial. Ophthalmology. 2016;123:484–91.
Queiroz de Paiva AR, Abreu de Azevedo Fraga L, Torres VLL. Surgical reconstruction of ocular surface tumors using fibrin sealant tissue adhesive. Ocul Oncol Pathol. 2016;2:207–11.
Bahar I, Weinberger D, Dan G, Avisar R. Pterygium surgery: fibrin glue versus vicryl sutures for conjunctival closure. Cornea. 2006;25:1168–72.
Hall RC, Logan AJ, Wells AP. Comparison of fibrin glue with sutures for pterygium excision surgery with conjunctival autografts. Clin Exp Ophthalmol. 2009;37:584–9.
Ratnalingam V, Keat Eu AL, Ng GL, Taharin R, John E. Fibrin adhesive is better than sutures in pterygium surgery. Cornea. 2010;29:485–9.
Sotozono C, Ang LPK, Koizumi N, Higashihara H, Ueta M, Inatomi T, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology. 2007;114:1294–302.
Kompella VB, Sangwan VS, Bansal AK, Garg P, Aasuri MK, Rao GN. Ophthalmic complications and management of Stevens-Johnson syndrome at a tertiary eye care centre in south India. Indian J Ophthalmol. 2002;50:283–6.
Weinberg DA, Tham V, Hardin N, Antley C, Cohen AJ, Hunt K, et al. Eyelid mucous membrane grafts: a histologic study of hard palate, nasal turbinate, and buccal mucosal grafts. Ophthal Plast Reconstr Surg. 2007;23:211–6.
Henderson HWA, Collin JRO. Mucous membrane grafting. In: Geerling G, Brewitt H, editors. Developments in ophthalmology. Basel: Karger; 2008. p. 230–42.
Araújo ALde, Ricardo JRdaS, Sakai VN, Barros JNde, Gomes JÁP. Impression cytology and in vivo confocal microscopy in corneas with total limbal stem cell deficiency. Arq Bras Oftalmol. 2013;76:305–8.
Fatima A, Iftekhar G, Sangwan VS, Vemuganti GK. Ocular surface changes in limbal stem cell deficiency caused by chemical injury: a histologic study of excised pannus from recipients of cultured corneal epithelium. Eye. 2008;22:1161–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pushker, N., Gorimanipalli, B., Sharma, N. et al. Mucous membrane grafting (fibrin glue vs. suture) for lid margin pathologies in Stevens–Johnson syndrome: randomized comparative study. Eye 35, 1985–1992 (2021). https://doi.org/10.1038/s41433-020-01203-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01203-4