Abstract
Purpose
To study the relationship between macular ganglion cell complex (GCC) thickness and visual field defects (VFD) caused by central nervous system (CNS) lesions in children and evaluate the possibility of predicting VFD according to GCC maps.
Methods
The GCC maps of a group of children with VFD due to CNS lesions with respect of the vertical meridian in at least one eye (study group), as well as of children with other neuro-ophthalmological problems and healthy children were presented to two masked evaluators, who were asked to predict the patients’ VFD on the basis of GCC damage: the evaluators classified VFD as normal, hemianopia (homonymous or heteronymous) or diffuse.
Results
Seventeen patients were included in the study group, with a median age of 12 years. Fifteen had brain tumours and two epilepsy. The mean MD of the affected hemifields was −26.00 dB (SD 7.89 dB) versus −5.51 dB (SD 3.52 dB) for the nonaffected hemifields, p < 0.001. The mean GCC thickness was of 56.04 μm (SD 11.95 μm) in the affected hemiretinas versus 74.31 μm (SD 10.64 μm) for the non-affected, p < 0.001. Kappa coefficients between VFD and those estimated by the evaluators were 0.705 and 0.658 (p < 0.001) for evaluators 1 and 2.
Conclusions
GCC thickness can reflect damage to the visual pathway and GCC maps may be useful to identify chiasmal and retrochiasmal lesions, since GCC atrophy in most of these cases respects the vertical meridian. GCC maps might be used as a surrogate marker for visual damage in patients unable to perform perimetry.
Similar content being viewed by others
Introduction
Bilateral heteronymous or homonymous hemianopia can develop due to several diseases of the central nervous system such as tumours, inflammatory or vascular diseases which damage the visual pathway. Hemianopia rarely appears in children; however, recognition of visual field defects is important to diagnose and monitor cerebral damage and to evaluate the possible repercussion on the child’s development. Unfortunately, children often do not recognise or complain of visual loss [1]. Evaluating only visual acuity (VA) would underestimate visual damage since it can be normal in the presence of visual field defects [2]. Often an anomalous position of the head, with the face turned towards the side of the hemifield defect may be the only sign in children with homonymous hemianopia [3].
The location and radiological features of the brain lesions cannot always predict visual damage, particularly in visual pathway glioma. The management of these lesions is difficult and is based on their clinical repercussion, i.e., the extent of visual damage and the potential for visual improvement [4]. Being able to detect early, subtle changes are essential because visual pathway glioma may not require chemotherapy (which may lead to severe side effects) unless visual loss or progression in visual field defects are demonstrated [2]. Automatic perimetry may sometimes be performed by children as young as 6 years-old [5, 6], but often even older children are unable to perform reliable visual fields.
Damage to the human central nervous system leads to retrograde trans-synaptic degeneration and when the visual pathway is involved, it often leads to axonal loss at the optic nerve and retinal ganglion cell loss at the retina. Optical coherence tomography (OCT) is capable of detecting axonal loss in the peripapillary area. However, when there is a decreased peripapillary retinal nerve fibre layer (pRNFL) thickness, the extent of damage to the visual pathway may preclude visual recovery after treatment [7]. Software developments now also allow OCT measurement of the ganglion cell layer in the macular area of the retina, which might be better correlated to visual field loss than RNFL thickness. OCT evaluation would be especially helpful in evaluating children who cannot perform visual fields, since it is a very fast test which requires minimum patient collaboration [8].
The aim of this study was to evaluate the relationship between reduction in macular ganglion cell complex (GCC) thickness as detected with spectral-domain OCT and visual field defects caused by central nervous system lesions in children, as well as to determine whether it is possible to predict visual field defects from GCC maps.
Patients and methods
Children with visual field defects due to brain lesions, with respect of the vertical meridian in at least one eye, who had been followed-up in the Neuro-ophthalmology Department between 2011 and 2016, were evaluated for inclusion in the study. To be included, two standard automated perimetry (Humphrey Field Analyser 750, Zeiss/Humphrey Systems, Dublin, Calif.) 24-2 tests, Swedish Interactive Test Algorithm (SITA) strategy had to be available with similar results in extension and severity of the visual defect. At least 6 months had to have elapsed between diagnosis of the brain tumour or its treatment and the first of these visual fields; a further 3 months were required between both fields in order to ensure that visual damage was stable. Only the second of these visual fields was included in the analysis. Visual fields were considered reliable when fixation losses and positive errors were <10%. Hemianopia was diagnosed following the criteria proposed by Thomas et al., when the threshold values showed depression of three or more contiguous points by 5 dB or more along the vertical meridian as compared with their mirror image points in one quadrant and depressed threshold values were present in the other quadrant of the same hemifield. Alternatively, the diagnosis was made if three points of the pattern deviation probability plot were depressed to 1% or lower probability level along the vertical meridian and as compared with their mirror image points in one quadrant and depressed threshold values were present in the other quadrant of the same hemifield [9, 10]. In order to simplify the evaluation, visual field defects were classified as normal, hemianopia or diffuse; and then as homonymous or heteronymous. The average mean deviation of each hemifield was calculated and recorded. When VA was less or equal to counting finger in one eye, the visual field was not tested and MD was defined as −30 dB for the purpose of analysis.
All patients also underwent an extensive ophthalmic evaluation including at least best-corrected VA, internal and external ocular motor examinations and anterior and posterior pole biomicroscopy and funduscopy.
Optical coherence tomography scanning was performed through dilated pupils using Cirrus-HD Model 4000 (Carl Zeiss Meditec Dublin, CA, USA). An internal fixation target was used whenever possible. To evaluate the pRNFL thickness and the GCC thickness, the optic disc cube protocol and the macular cube 512 × 128 protocols were used. The GCC analysis algorithm divides the macula into six equal sectors across a horizontal meridian. In normal subjects all segments should have a similar thickness. To establish a thickness measurement for the nasal macula, the superonasal sector and the inferonasal sector were averaged, and similarly the temporal thickness was calculated as the average of the superotemporal and inferotemporal sectors. (Fig. 1). The inferior and superior segments were left out of the analysis as they include both crossed and uncrossed fibres [11].
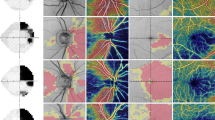
a Visual field, and b Optical coherence tomography analysis of the ganglion cell complex (GCC) of the right eye of a 13 year old girl with a middle line glioma. Right eye hemianopia (the left eye had a severe diffuse defect). The colour coded map of the GCC reflects the intense ganglion cell loss in the nasal hemiretina, reflecting the temporal visual field loss. Both evaluators predicted the presence of the hemianopia when presented with the GCC analysis. The red ovals show how the superotemporal and inferotemporal sectors were averaged to calculate the mean GCC thickness of the temporal retina (83 µm) and the superonasal and inferonasal to calculate the mean GCC thickness of the nasal hemiretina (48.5 µm)
The GCC maps were presented to two neuro-ophthalmologists (IC and MG) who were asked to predict the visual field defects the patients had on the basis of GCC damage. Maps from the children included in the study (with visual field defects caused by central nervous system lesions) were presented mixed with maps from a control group. This group consisted of 18 subjects: 5 healthy volunteers and 13 subjects diagnosed with other neuro-ophthalmic diseases such as optic neuritis, optic nerve drusen or intracranial hypertension, of which only ten eyes had visual field defects.
The study was approved by our institutional review board and adhered to the tenets of the Declaration of Helsinki. Since this was an exploratory study, no power calculation was performed prior to initiation. Statistical analysis was performed using the Statistical Package for Social Sciences software (version 20.0; SPSS, Inc, Chicago, IL). Kappa coefficients were calculated to determine the correlation between the responses of the evaluators and the actual visual field defects of patients. The correlation is considered to be low if the Kappa coefficient is <0.4, good if it is between 0.4 and 0.75 and excellent if it is >0.75 (Fig. 2).
a Visual fields, and b Optical coherence tomography analysis of the ganglion cell complex (GCC) of both eyes of a 10 year old boy with a craniopharyngioma. Visual acuity was 20/30 in the right eye and counting fingers in his left eye. Visual fields showed a temporal hemianopia in the right eye and a severe, diffuse defect in the left eye. Both evaluators predicted there would be a diffuse defect in both eyes when presented with the CGG analysis, due to the diffuse GCC loss. The mean GCC for the right eye is above the cutoff point of 53 µm, which increases the probability of finding an hemianopia instead of a diffuse defect
Results
Seventeen patients younger than 18 years at the time visual field and OCT examination were performed were included in the study. One patient with a homonymous defect due to ischaemic damage was excluded because criteria for hemianopia were not fulfilled. There were eight girls (47%) and nine boys (53%), with a median age of 12 years (range from 5 to 17 years). Brain tumour was the most frequent pathology with 15 cases (88%): six craniopharyngiomas, six visual pathway gliomas, one ependymoma, one pituitary adenoma and one choroid plexus papilloma. The remaining two cases were caused by sequels of disseminated acute encephalitis and shaken baby syndrome with occipital cortex involvement.
Only five children had a visual acuity of 20/20 (Snellen equivalent) in both eyes (29%); six had a VA of 20/20 in one eye with decreased visual acuity in the fellow eye (35%), ranging from amaurosis to 20/30; two (12%) had a bilateral moderate reduction (between 20/25 and 20/40) and four (24%) had a visual acuity of 20/40 or less in both eyes. Five children had strabismus; four with reduced visual acuity in at least one eye presented exotropia (23.5%) and one child with bilateral normal VA, esotropia (6%). Two other children had nystagmus (12%).
Nine patients had a homonymous hemianopia (53%), seven had a temporal defect in one eye and a diffuse defect in the contralateral eye (41%) and one case, an heteronymous hemianopia (6%). Mean deviation (MD) ranged from −3.79 to −34.73 dB, with a mean MD for the 34 eyes of −18.03 dB (SD 7.72 dB).
Good quality scans of the optic nerve could be obtained in all except one eye. Mean pRNFL thickness for the 33 eyes was 66.88 μm (SD 12.47 μm), with a range between 42 and 90 μm. In two eyes, the OCT software did not accurately segment the macular region and GCC measurements were unreliable. The mean GCC thickness was 62.44 μm (SD 11.26 μm), with a range between 43 and 92 μm in the remaining 31 eyes. Table 1 shows the values for pRNFL thickness and GCC, with significant differences in mean MD and OCT thicknesses between eyes with hemianopia and diffuse defects.
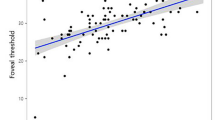
A more detailed analysis was performed in eyes with hemianopia (27 eyes), in order to compare the affected with the unaffected hemifields. The mean MD of the affected hemifields was −26.00 dB (SD 7.89 dB) and −5.51 dB (SD 3.52 dB) for the non-affected hemifields. The difference was statistically significant (p < 0.001, Wilcoxon test). The mean GCC thickness of the affected hemiretinas (nasal or temporal macula) was 56.04 μm (SD 11.95 μm) and 74.31 μm (SD 10.64 μm) for the non-affected hemiretinas. The difference was statically significant (p < 0.001, Wilcoxon test). No significant correlations were found between MD and mean GCC thickness in any hemifield.
We calculated kappa coefficients to determine the correlation between the responses of the neuro-ophthalmologist evaluators and the actual visual field defects of patients. Between evaluator 1 and actual defects the kappa coefficient was 0.705 (p < 0.001); between evaluator 2 and actual defects the kappa coefficient was 0.658 (p < 0.001) and between the two evaluators the kappa coefficient was 0.841 (p < 0.001). When taking into account only cases with chiasmatic or retrochiasmatic lesions, the most frequent mistake was made in the prediction of diffuse defects instead of the real hemianopias: evaluator 1 predicted 11 diffuse defects and evaluator 2, 10 diffuse defects, when there were only seven diffuse defects, with three hemianopias.
Overall mean GCC thickness was significantly different in eyes with hemianopia and eyes with a diffuse defect [65.26 μm (SD 9.80 μm) versus 47.20 μm (SD 3.70 μm), p < 0.001]. Its ability to discriminate hemianopia from diffuse defect was calculated by the area under the curve of the ROC curve (ROC = 0.978, p < 0.001 confidence interval 95: 0.93–1.00). According to the Jouden Index, a mean GCC thickness of 53 μm would be the best discriminating cut-off point, with a sensitivity of 93% and a specificity of 100%.
Discussion
In glaucomatous optic neuropathy, damage to the pRNFL can precede future perimetric damage by up to 5 years, and therefore, OCT has been recognised as a useful diagnostic tool in detecting early glaucoma [12, 13]. However, in compressive optic neuropathy, the relationship between OCT measurements and visual damage is more complex. Damage to the visual pathway has been shown to lead to transynaptic retrograde degeneration (TSRD), which may be detected with OCT evaluation both of the pRNFL and GCC [14]. Acute or rapidly progressive chiasmal compression may produce axonal dysfunction with visual field deficits such as bitemporal hemianopias before structural damage can be demonstrated. Thus, pRNFL values within normal values in patients with visual fields due to chiasmal tumours have been shown to predict visual improvement after treatment. This predictive role has been established in adults with pituitary adenoma [15] and craniopharingioma [16]. Therefore, perimetry would seem to be better than OCT evaluation of the pRNFL to detect early compression of the visual pathway in order to achieve visual improvement after treatment, both after the initial diagnosis or after recurrences. Most of these tumours cannot be completely resected (especially in the case of childhood craniopharyngiomas) and follow-up is critical to detect visual impairment which is an important factor to decide if further treatment is necessary. But children are often unable to perform reliable visual fields and it is necessary to search for an alternative surrogate marker for visual loss for both diagnosis and follow-up. Furthermore, it seems that in chronic compressive neuropathies, ganglion cell loss can take place before visual field deficits [17,18,19].
Several studies suggest that GCC thinning is more apt to occur than pRNFL thinning following damage to the visual pathway and that it may reflect damage earlier than the pRNFL. Blanch et al. reported seven patients with radiologic compression of the chiasm by a sellar tumour, in whom OCT-GCC analysis detected damage without visual field defects apparent on standard automated visual field testing; three of these patients had normal PRNFL thicknesses [17]. Tieger et al. also reported six cases of patients with sellar tumours without visual field loss, with more significant GCC than RNFL thinning, as well as cases with binasal GCC loss without RNFL thinning on OCT [18]. Persistent GCC thinning with improvement in visual fields was noted in seven of eight patients who underwent surgical decompression (after decompression, 93% of eyes had nasal GCC loss postoperatively and 87% experienced VF improvement) [18]. After decompression of the optic chiasm, physiological conduction block is reversed in axons that were still viable and thus visual function might improve with stable GCC and pRNFL. Yum et al. evaluated the detection rate of abnormal colour codes on GCC sector maps in 46 patients with pituitary adenoma; an abnormal yellow colour code was found in the inferonasal sector 34 (73.9%), followed by the superonasal 29 (63.0%) and inferior 29 (63.0%) sectors. Furthermore, analysis of the GCC sector maps revealed that 25% of preperimetric eyes in patients with pituitary adenomas had an abnormal yellow or red colour code in the inferonasal sector [19].
It seems therefore that GCC thickness is affected early in the course of compressive optic neuropathy and that it may be easier to suspect chiasmal and retrochiasmal damage when evaluating GCC maps than when evaluating pRNFL as the atrophy respects the vertical meridian in both eyes. Conversely, the more complex pattern of pRNFL thinning in chiasmal and retrochiasmal pathologies must be carefully analysed to differentiate TSRD from optic atrophy due to other causes [20, 21]. Therefore, our purpose was to evaluate the relationship between topographic reduction in GCC thickness and visual field defects caused by central nervous system lesions in children, to explore whether the GCC could provide a valid surrogate marker for visual loss when visual fields are unavailable. The evaluation of the colour maps is especially important in children since there is currently no available normality data.
Brain tumours were the most common cause of hemianopia in this paediatric case series, mainly craniopharyngiomas and optic pathway gliomas. Children were included if they presented hemianopia in at least one eye and it is noteworthy that 41% had a diffuse defect in the fellow eye. However, visual acuity was below 20/40 in both eyes in only one out of four cases. This confirms that visual acuity underestimates visual damage when compared with perimetry. As might be expected, visual field and OCT variables were significantly lower in the affected hemifield than in the unaffected one, although they did not show a significant correlation. Herro et al. studied 17 eyes of nine adults with visual field defects secondary to ischaemic cortical injury with the Cirrus SD-OCT device. They did not find a statistically significant difference between affected and unaffected pRNFL. However, there was a significant difference in GCC layer reduction between the affected (69.9 μm) and unaffected (75.2 μm) sides (P = 0.029) [11). Goto et al. detected a reduction of the pRNFL thickness corresponding to the hemianopic visual field loss due to acquired post-geniculate visual pathway lesions [22]. The lack of a correlation between visual field damage and OCT parameters might be due to the different time elapsed between the damage to the visual pathway and the moment of visual field and OCT examination. Jindhara et al. found that the speed of RTSD is greatest within the first 2 years, occurring in the pRNFL at a rate of 4.4 μm/year. Clear evidence of RTSD typically becomes apparent by 1 year, and progression may continue over the course of a decade [23].
As regards studies performed in children, Gu et al. studied the relationship between GCC thickness and vision loss in 26 children with optic pathway gliomas. They compared 31 normal eyes with 16 eyes with decreased visual acuity or visual field defects. All the pRNFL and GCC thickness measures were significantly different between normal and affected eyes, although the highest area under the curve was obtained for the fifth percentile GCC of the inner macula. The positive predictive value of the centre (3 mm) macula demonstrated that 88.9% of those with decreased GCC thickness had visual loss. They concluded that if children with normal vision demonstrate a progressive decline in their GCC thickness, this may be an indication to initiate an early treatment [2].
Thus, it seems that there is enough evidence (from ours and previous studies) to support that GCC thickness may be a valid surrogate marker for visual loss in patients unable to perform visual fields, such as young children. However, the utility of GCC thickness to predict the extent of visual field damage in clinical practice has not been tested yet, to the best of our knowledge. In this study we found that the evaluation of the GCC map by an experienced neuro-ophthalmologist may be quite sensible for detecting visual damage; in fact the most frequent mistake was anticipating diffuse defects instead of the real hemianopias. If an evaluation of the map was to be combined with an analysis of the mean GCL-IPL thickness, the prediction of visual field damage might improve. Therefore, GCC evaluation might be useful both for diagnosis and for follow-up of children with suspected central nervous system pathology who are unable to undergo visual field testing. However, visual field testing should be performed whenever possible, since depending on the clinical situation, both OCT and perimetry have advantages and disadvantages for the detection of chiasmal compression [24].
The main limitation of our study is that it is a small, retrospective series and the results must be considered with caution. The lack of a correlation between GCC thickness and visual field mean deviation might be due to the less number of patients included, i.e., our study might be underpowered. It included children with very different brain lesions and with different time-courses. Another limitation is that we included only patients with a stable clinical situation and therefore it is uncertain whether in a clinical setting, GCC analysis would be truly useful to suspect diagnosis of CNS or progression of the lesion if visual fields are unavailable. However, it seems clear that GCC can reflect damage to the visual pathway and that it’s especially useful to identify chiasmal and retrochiasmal lesions, since GCC atrophy in these cases respects the vertical meridian. Thus, it might be used as a surrogate marker to detect visual damage in patients unable to perform visual fields.
Summary
What was known before
-
Several studies have shown that perimetry would be better than OCT evaluation of the peripapillary retinal nerve fibre layer (pRNFL) to detect early compression of the visual pathway.
-
However, children are often unable to perform reliable visual fields and it is necessary to search for an alternative surrogate marker for visual loss for both diagnosis and follow-up.
What this study adds
-
Damage to the visual pathway has been shown to lead to transynaptic retrograde degeneration, which may be detected with OCT evaluation both of the pRNFL and ganglion cell complex (GCC).
-
Therefore, our purpose was to evaluate the relationship between topographic reduction in GCC thickness and visual field defects caused by central nervous system lesions in children, to explore whether the GCC could provide a valid surrogate marker for visual loss when visual fields are unavailable.
References
Mccrea HJ, George E, Settler A, Schwartz TH, Greenfield JP. Pediatric suprasellar tumors. J Child Neurol. 2016;31:1367–76.
Gu S, Glaug N, Cnaan A, Packer RJ, Avery RA. Ganglion cell layer – inner plexiform layer thickness and vision loss in young children with optic pathway gliomas. Investig Ophthalmol Vis Sci. 2014;55:1402–8.
Paysse EA, Coats DK. Anomalous head posture with early-onset homonymous hemianopia. J AAPOS. 1997;1:209–13.
Aquilina K, Daniels DJ, Spoudeas H, Phipps K, Gan H-W, Boop FA. Optic pathway glioma in children: does visual deficit correlate with radiology in focal exophytic lesions? Child's Nerv Syst. 2015;31:2041–9.
Patel DE, Cumberland PM, Walters BC, Russell-Eggitt I, Rahi JS, OPTIC study group. Study of optimal perimetric testing in children (OPTIC): feasibility, reliability and repeatability of perimetry in children. PLoS One. 2015;10:e0130895.
Han S, Baek S-H, Kim US. Comparison of three visual field tests in children: frequency doubling test, 24-2 and 30-2 SITA perimetry. Semin Ophthalmol. 2016;12:1–4.
Danesh-Meyer HV, Wong A, Papchenko T, Matheos K, Stylli S, Nichols A, et al. Optical coherence tomography predicts visual outcome for pituitary tumors. J Clin Neurosci. 2015;22:1098–104.
Barrio-Barrio J, Noval S, Galdós M, Ruiz-Canela M, Bonet E, Capote M, et al. Multicenter Spanish study of spectral-domain optical coherence tomography in normal children. Acta Ophthalmol. 2013;91:e56–63.
Thomas D, Thomas R, Muliyil JP, George R. Role of frequency doubling perimetry in detecting neuro-ophthalmic visual field defects. Am J Ophthalmol. 2001;131:734–41.
Noval S, Contreras I, Rebolleda G, Muñoz-Negrete FJ, Ruiz, de Zárate B. A comparison between Humphrey and frequency doubling perimetry for chiasmal visual field defects. Eur J Ophthalmol. 2005;15:739–45.
Herro AM, Lam BL. Retrograde degeneration of retinal ganglion cells in homonymous hemianopsia. Clin Ophthalmol. 2015;9:1057–64.
Quigley HA, Katz J, Derick RJ, Gilbert D, Sommer A. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992;99:19–28.
Liu C-H, Chang SHL, Wu S-C. Regional relationship between macular retinal thickness and corresponding central visual field sensitivity in glaucoma patients. J Ophthalmol. 2017:3720157. https://doi.org/10.1155/2017/3720157.
Danesh-Meyer HV, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Investig Ophthalmol Vis Sci. 2008;49:1879–85.
Mediero S, Noval S, Bravo-ljubetic L, Carceller F. Visual outcomes, visual fields, and optical coherence tomography in paediatric craniopharyngioma. Neuroophthalmology. 2015;39:132–9.
Blanch RJ, Micieli JA, Oyesiku NM, Newman NJ, Biousse V. Optical coherence tomography retinal ganglion cell complex analysis for the detection of early chiasmal compression. Pituitary. 2018;21:515–23.
Tieger MG, Hedges TR III, Ho J, Erlich-Malona NK, Vuong LN, Athappilly GK, et al. Ganglion cell complex loss in chiasmal compression by brain tumors. J Neuroophthalmol. 2017;37:7–12.
Yum HR, Park SH, Park HY, Shin SY. Macular ganglion cell analysis determined by cirrus HD optical coherence tomography for early detecting chiasmal compression. PLoS ONE. 2016;11:e0153064.
Omodaka K, Yokoyama Y, Shiga Y, Inoue M, Takahashi S, Tsuda S, et al. Topographical correlation between macular layer thickness and clockwise circumpapillary retinal nerve fiber layer sectors in patients with normal tension glaucoma. Graefe’s Arch Clin Exp Ophthalmol. 2015;40:744–51.
Kanamori A, Nakamura M, Yamada Y, Negi A. Spectral-domain optical coherence tomography detects optic atrophy due to optic tract syndrome. Graefe's Arch Clin Exp Ophthalmol. 2013;251:591–5.
Yamashita T, Miki A, Iguchi Y, Kimura K, Maeda F, Kiryu J. Reduced retinal ganglion cell complex thickness in patients with posterior cerebral artery infarction detected using spectral-domain optical coherence tomography. Jpn J Ophthalmol. 2012;56:502–10.
Goto K, Miki A, Yamashita T, Araki S, Takizawa G, Nakagawa M, et al. Sectoral analysis of the retinal nerve fiber layer thinning and its association with visual field loss in homonymous hemianopia caused by post-geniculate lesions using spectral-domain optical coherence tomography. Graefe’s Arch Clin Exp Ophthalmol. 2016;254:745–56.
Jindahra P, Petrie A, Plant GT. The time course of retrograde transsynaptic degeneration following occipital lobe damage in humans. Brain. 2012;135:534–41.
Horton JC. Invited commentary: ganglion cell complex measurement in compressive optic neuropathy. J Neuroophthalmol. 2017;37:13–15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noval, S., Henríquez-Recine, M.A., Contreras, I. et al. Macular ganglion cell complex thinning in children with visual field defects due to central nervous system pathology. Eye 34, 1570–1576 (2020). https://doi.org/10.1038/s41433-019-0650-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-019-0650-5