Abstract
Objective
To investigate complement activation in aqueous humour of patients with early, intermediate and neovascular age-related macular degeneration (AMD).
Patients and methods
Aqueous humour of 79 AMD patients (early, intermediate and neovascular) and 77 age-matched controls was prospectively collected. The levels of the complement protein 3 (C3), activation products complement factor 3a (C3a) and Ba, C3b/iC3b, complement factors B, H and I (CFB, CFH and CFI), and total protein concentration were measured. Data were modelled using covariate analysis to assess the impact of age and glaucoma status of patients and total protein concentration of samples on complement protein concentration across groups.
Results
C3a concentration was significantly increased in the aqueous humour of early (p = 0.016), intermediate (p = 0.003) and neovascular (p = 0.018) AMD patients, whilst C3 concentration was significantly increased in early AMD patients only (p = 0.019). Levels of CFB and CFH were significantly increased in the aqueous humour of neovascular AMD patients (p = 0.023 and p = 0.018, respectively).
Conclusions
Our findings provide evidence for early local complement dysregulation in AMD patients, suggesting that complement pathway inhibition may be a clinically relevant intervention for early stages of AMD.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD), a leading cause of vision loss in the elderly population, has been associated with dysregulation of the alternative complement pathway [1]. Complement proteins have been identified in drusen, the hallmark deposits exhibited in AMD patients [2, 3]. Furthermore, genetic variations in genes encoding for the complement pathway, including Complement Factor H (CFH), Complement Factor B (CFB) and C3, confer significantly increased risk for AMD initiation and progression [4, 5].
Recent studies analysing the aqueous humour of neovascular AMD patients reported statistically significant elevation of the complement activation products Ba and C3a, and a trend towards elevated complement regulatory proteins CFH and CFI, suggesting a role for local complement activation in advanced disease [6]. However, studies investigating complement pathway activation in earlier stages of AMD have not been carried out. Such studies could enable the development of targeted therapies for earlier clinical intervention in AMD patients, and would facilitate clinical translation of disease biomarkers for early disease detection and risk staging. We therefore carried out aqueous humour analyses to evaluate the association of alternative pathway-associated complement proteins with early and intermediate AMD. We observed that complement factor C3a, was elevated in both early and intermediate stages of AMD, suggesting that local complement activation occurs prior to development of advanced forms of AMD. Therefore, complement pathway inhibition may be a clinically relevant intervention for early and intermediate stages of AMD.
Materials and methods
Study population
This prospective study included clinical data of 156 participants (17 early AMD, 27 intermediate AMD and 35 nAMD patients and 77 age-matched controls) from the Department of Ophthalmology, University Hospital of Cologne. The study was performed in accordance with the tenets of the Declaration of Helsinki and the Medical Research Involving Human Subjects Act (WMO) and was approved by the local ethics committee of the University Hospital in Cologne. Written informed consent was obtained from all participants.
In addition to patient age, gender and AMD status, information regarding glaucoma, hypertension and diabetes status was also collected for all patients.
Inclusion and exclusion criteria
AMD patients and controls undergoing cataract surgery or intravitreal injections (IVI) with anti-vascular growth factor (anti-VEGF) agents (bevacizumab, ranibizumab or aflibercept) within clinical routine were included. The clinical decision of cataract surgery or IVI injection was independent of participation in this study. All subjects with age > = 60 undergoing cataract surgery and IVI injections between January 2016 and November 2016 in Department of Ophthalmology, University of Cologne were asked for participation in this study.
Subjects with retinal pathologies other than AMD such as diabetic retinopathy/maculopathy, high myopia (≥−6 dioptres), macular hole, severe macular pucker and subjects with any previous ophthalmic surgery during the last 6 months (except IVI with anti-VEGF) were excluded. Also, subjects undergoing IVI with steroids within last 6 months as well as subjects with IVI within less than 4 weeks were excluded. Additional exclusion criteria were use of local or systemic steroids/immunosuppressant agents, subjects with diseases resulting in inflammation or haemorrhage in the study eye and any systemic diseases potentially inducing the complement system such as autoimmune diseases, cancer or infectious diseases. Moreover, patients with geographic atrophy (GA) were not included in this study.
Grading procedure
AMD grading was performed by two graders (VS, LA) based on digital colour fundus photographs (FP; Canon UVI fundus camera; Canon, Tokyo, Japan) and Spectral Domain Optical coherence tomograms (SD-OCT, Spectralis HRA system; Heidelberg Engineering, Heidelberg, Germany) as in [7]. Fluorescein angiography (FA) was performed only in cases with questionable neovascular activity. In cases of disagreement, the graders discussed the cases until a consensus was reached.
Controls had no drusen and no pigmentary changes in both eyes. Early AMD was classified by the presence of at least ten small drusen (<63 μm) and pigmentary changes or the presence of 1–14 intermediate (63–124 μm) drusen in the Early Treatment Diabetic Retinopathy Study (ETDRS) grid. Intermediate AMD was classified by the presence of ≥15 intermediate drusen or any large drusen (≥125 μm) within ETDRS grid. Cases with leakage in FA, intra-/subretinal haemorrhage and intra-/subretinal fluid in SD-OCT were graded as nAMD. GA was defined as a sharply demarcated area of retinal pigment epithelium depigmentation ≥175 µm with increased visibility of choroidal vessels.
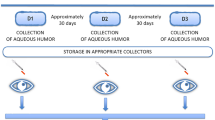
Sample collection
Undiluted samples of aqueous humour were acquired before any other surgical manipulation during the cataract operation. An aliquot of up to 0.1 ml of aqueous humour was collected via limbal paracentesis using a 30-gauge needle connected to an insulin syringe. Care was taken not to contaminate the sample with blood. Samples were transferred to sterile polypropylene tubes immediately after collection and stored at −80 °C until analysis was performed.
Measurement of complement and protein levels
As patient samples were measured in multiple assays, prior to starting, each sample was diluted to create stocks at 1:30, 1:100 and 1:1000 dilutions thereby minimizing pipetting errors between analyses. C3a and Ba concentrations in aqueous humour samples were measured using C3a or Ba Microvue-plus ELISA (Quidel, San Diego, CA, USA). Assay steps were performed according to the manufacturer’s instructions. Absorbance was read at an optical density of 450 nm on a FlexStation 4 spectrophotometer (Molecule Devices). Absorbance values for the standard curve were fitted using a 5 parameter logistic model and XLfit 5.5 software (IDBS). C3a and Ba concentrations of patient samples were interpolated from the standard curve and corrected for the dilution factor.
C3, C3b/iC3b, CFB, CFH, CFI concentrations were measured using Milliplex Human Complement Panels 1 and 2 (Millipore). Assay steps were performed according to the manufacturer’s instructions. Quantification was performed using a Luminex 200 device (Thermo Fisher Scientific) and analysis of protein concentration by standard curve interpolation performed using LiquiChip Analyzer Version 1.0.6.27262 (Luminex).
Total protein concentration in aqueous humour samples was determined using the Quant-iT™ Protein Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Fluorescence was read at 470/570 nm using a FlexStation 4 spectrophotometer (Molecule Devices). Protein concentration of aqueous humour samples was interpolated from the protein concentration standard curve fitted using a 5 parameter logistic model and XLfit 5.5 software (IDBS) and corrected for dilution factor.
Samples were measured with all assays unless sample volume was insufficient. Results with values below the limit of quantification of the assay were not excluded from the analysis.
Statistical analysis
As this is a pilot study and no boundary values for local complement values exist in these patient populations, power calculations could not be performed previously. All complement protein concentrations were log-transformed before statistical analysis. A linear model was used to test the association between complement protein concentrations and disease status. One model was built for each protein using covariates to account for age, gender, total protein concentration and glaucoma status. Covariates were selected after exhaustive search based on information criterion as described in [8]. Protein concentrations which were below the limit of quantification of the assay were imputed with a value randomly drawn (using a beta distribution with a = 10 and b = 1) between 0 and the lower limit of quantification of the corresponding assay. For each complement protein, β coefficient of association and 95% confidence intervals were computed for the different AMD disease status. A two-tailed t-test was performed to test the null hypothesis (β = 0, i.e. no association between complement protein concentrations and AMD disease status). A p value below 5% was considered as significant.
All analyses were performed with R version 3.5.1.
Results
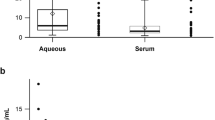
One-hundred and fifty-six participants with protein data were included in the analysis. Patient demographics are shown in Table 1. Control patients were significantly younger than AMD patients (early, intermediate or neovascular) and presence of glaucoma was significantly enriched in the control and intermediate-AMD groups (Table 1). There were no significant differences in the distribution of gender and arterial hypertension status across groups. Total protein and complement factor concentrations in aqueous humour of AMD patients and controls are shown in Table 2. Significant positive correlation was observed between total protein levels and complement protein concentrations of C3b/iC3b, CFB, Ba, CFH, CFI, while only a positive non-significant trend was observed for C3 and C3a. Age was not correlated with the different complement proteins. Gender was associated significantly only with C3b/iC3b and CFI. Glaucoma status was significantly associated only with C3b/iC3b, CFB, CFH and CFI.
Complement component C3 is activated by proteolytic cleavage by C3 convertases of the alternative (C3bBb) or classical and lectin (C4b2a) pathways [9,10,11]. C3 cleavage releases C3a and C3b, which can be further processed to generate iC3b, an inactive version of C3b [12]. Confirming previous observations [6] C3a, a marker of C3 activation, was significantly increased in the aqueous humour of neovascular AMD patients (p value = 0.018). C3a protein concentration was also significantly elevated in the aqueous humour of patients with early (p value = 0.016) and intermediate (p value = 0.003) AMD, compared to control patients without AMD (Table 2). Full-length C3 protein was elevated in aqueous humour of patients with early AMD (p = 0.019) (Table 2), but not in samples from patients with intermediate or neovascular AMD. Levels of C3b, iC3b, active or inactive versions of C3b respectively, were unchanged across groups (Table 2).
CFB is a component of the alternative pathway of complement activation, which can be cleaved by CFD to produce Ba and Bb. Bb is a serine protease which forms part of the alternative pathway C3 convertase (C3bBb), whilst Ba is a non-catalytic activation product [9]. Total CFB levels were significantly elevated in the neovascular AMD group only (p value = 0.023) (Table 2) whilst levels of Ba, a marker of CFB activation, were unchanged across the patient groups.
We also measured concentrations of the complement negative regulators CFH and CFI [9]. CFH was significantly increased in the aqueous humour of neovascular AMD patients, confirming a trend previously observed in studies of these patients [6] (p = 0.018) (Table 2). Elevated CFH levels were not observed in those samples taken from patients with early or intermediate AMD. Levels of CFI were unchanged across groups (Table 2).
Discussion
Dysregulation of complement, in particular the alternative pathway, has been strongly implicated in the pathogenesis of AMD by extensive genome wide association studies [13]. In this study we measured levels of total C3, C3 activation products and complement regulatory proteins in the aqueous humour of AMD patients and controls without AMD. Our statistical analysis adjusted for non-equivalent frequency of glaucoma-positive status across the patient groups (Table 1), allowing differences in complement protein concentration at progressive stages of AMD to be revealed. By demonstrating increased concentration of C3a in early and intermediate AMD patients, our results provide evidence for early local activation of complement during the pathogenesis of AMD. These data have important consequences for the early detection and therapeutic targeting of AMD.
The human RPE-choroid complex expresses the majority of classical and alternative complement cascade components [11] and these proteins have been detected in drusen and sub-RPE deposits in AMD [2, 3, 14]. Data from genetic studies [13, 15] in combination with biochemical characterization of the RPE-choroidal complex [16] have led to the hypothesis that local alternative pathway complement activation contributes to cellular dysfunction and disease sequelae in AMD [17, 18]. In early AMD samples in our study, full-length C3 was elevated in aqueous humour, suggesting that increased production of C3, perhaps as a consequence of ongoing cellular stress [19] may contribute to initial complement activity. More significantly, we observed changes in aqueous C3a level, the activation product of C3, in both early and advanced stages of AMD, indicating complement activation.
C3a has important roles within the immune system as an anaphylatoxin [12]. It promotes recruitment and activation of phagocytic immune cells [12] and production of pro-inflammatory cytokines such as TNFα and IL-1β by binding to its C3aR receptor [20]. However, C3a has also been suggested to play a distinct role in the pathogenesis of AMD, by binding to C3aR expressed on RPE cells and increasing sub-RPE deposit formation [19, 21, 22], one of the markers of early AMD [23]. C3a has also been implicated in the induction of VEGF production by RPE [24], an important pathomechanism in advanced neovascular AMD. In our study, C3a elevation in neovascular AMD patient samples was accompanied by a significant increase in levels of CFB, which may promote increased activity of the alternative pathway at advanced stages of the disease. CFH was also elevated in neovascular AMD aqueous humour, suggesting that regulatory mechanisms may be induced at later stages of the disease to compensate for the deleterious effects of complement activation. However, as high C3a level persists alongside CFH upregulation, these mechanisms are unable to halt complement activation and hence ongoing disease pathophysiology. By providing evidence for the presence of C3a and hence complement activation in both early and advanced AMD, our data give support to the hypothesis that complement activation plays a central role in AMD pathophysiology. Consequently, complement targeting may be an important interventional mechanism for treatment of both the early and late stages of the disease.
Beyond therapeutic intervention, our data suggest that detection of proteins such as C3a in aqueous humour may be a suitable approach to aid diagnosis of the early stages of AMD. Such diagnostic tools and biomarkers are needed in order to enable study of disease progression and also to target future therapies to the correct patient groups. This study utilized a relatively small number of patient samples, follow-up studies should analyse larger cohorts and cohorts recruited in different geographical locations in combination with longitudinal characterization of disease progression. Such analyses will be important to improve our understanding of which patient phenotypes or biomarkers could be utilized as diagnostic tools to facilitate early clinical intervention. Highlighting the importance of repeating analyses in different sample sets and larger patient populations, in particular if biomarker-led diagnostic approaches are considered, in this cohort we were unable to reproduce previous observations showing that the CFB activation product Ba is increased in neovascular AMD samples [6]. To complete our investigation, it would be particularly interesting to investigate the complement protein milieu and activation profile in patients with GA, in order to understand whether patient biomarkers differ in those patients who progress to either advanced form of AMD.
In conclusion our study clearly indicates increased complement activation in the aqueous humour of early and intermediate AMD patients, and confirms previous observations in patients with neovascular AMD. These data have important implications when considering the treatment or biomarker-led detection of early AMD.
Summary
What was known before
-
Genetic variations in genes encoding for the complement pathway confer significantly increased risk for AMD initiation and progression. Levels of C3a and Ba are elevated in the aqueous humour of patients with advanced neovascular AMD.
What this study adds
-
Complement activation occurs early during the pathogenesis of AMD. C3a can be detected in the aqueous humour of early and intermediate AMD patients. C3a could be a useful biomarker or target for intervention in AMD.
References
Zipfel PF, Lauer N, Skerka C. The role of complement in AMD. Adv Exp Med Biol. 2010;703:9–24. https://doi.org/10.1007/978-1-4419-5635-4_2. PubMed PMID: 20711704
Crabb JW. The proteomics of drusen. Cold Spring Harb Perspect Med. 2014;4:a017194 https://doi.org/10.1101/cshperspect.a017194. PubMed PMID: 24799364; PMCID: 4066642
Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:14682–7. https://doi.org/10.1073/pnas.222551899. PubMed PMID: 12391305; PMCID: 137479
Cooke Bailey JN, Pericak-Vance MA, Haines JL. Genome-wide association studies: getting to pathogenesis, the role of inflammation/complement in age-related macular degeneration. Cold Spring Harb Perspect Med. 2014;4:a017186 https://doi.org/10.1101/cshperspect.a017186. PubMed PMID: 25213188; PMCID: 4292090
Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 2005;308:419–21. https://doi.org/10.1126/science.1110359. PubMed PMID: 15761120
Schick T, Steinhauer M, Aslanidis A, Altay L, Karlstetter M, Langmann T, et al. Local complement activation in aqueous humour in patients with age-related macular degeneration. Eye. 2017;31:810–3. https://doi.org/10.1038/eye.2016.328. PubMed PMID: 28128795; PMCID: 5437332
Ristau T, Ersoy L, Lechanteur Y, den Hollander AI, Daha MR, Hahn M, et al. Allergy is a protective factor against age-related macular degeneration. Investig Ophthalmol Vis Sci. 2014;55:210–4. PMID: 24235017
Miller AJ. Subset selection in regression. 2nd ed. Boca Raton, FL.; London: CRC; 2002.
Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part I—molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262 https://doi.org/10.3389/fimmu.2015.00262. PubMed PMID: PMC4451739
Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, Lambris JD. Novel mechanisms and functions of complement. Nat Immunol. 2017;18:1288 https://doi.org/10.1038/ni.3858
Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. https://doi.org/10.1016/j.preteyeres.2009.11.003. PubMed PMID: PMC3641842
Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343:227–35. https://doi.org/10.1007/s00441-010-1034-0. Epub 2010/09/15PubMed PMID: 20838815; PMCID: PMC3097465
Fritsche LG, Igl W, Bailey JN, Grassmann F, Sengupta S, Bragg-Gresham JL, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48:134–43. https://doi.org/10.1038/ng.3448. PubMed PMID: 26691988; PMCID: 4745342
Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–96. https://doi.org/10.1006/exer.2001.1094. Epub 2002/02/16PubMed PMID: 11846519
Seddon JM, Yu Y, Miller EC, Reynolds R, Tan PL, Gowrisankar S, et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013;45:1366–70. https://doi.org/10.1038/ng.2741. Epub 2013/09/17PubMed PMID: 24036952; PMCID: PMC3902040
Loyet KM, DeForge LE, Katschke JKJ, Diehl L, Graham RR, Pao L, et al. Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Investig Ophthalmol Vis Sci. 2012;53:6628–37. https://doi.org/10.1167/iovs.12-9587
Toomey CB, Kelly U, Saban DR, Bowes Rickman C. Regulation of age-related macular degeneration-like pathology by complement factor H. Proc Natl Acad Sci USA. 2015;112:E3040–9. https://doi.org/10.1073/pnas.1424391112. Epub 2015/05/21PubMed PMID: 25991857; PMCID: PMC4466717
Toomey CB, Johnson LV, Bowes Rickman C. Complement factor H in AMD: bridging genetic associations and pathobiology. Prog Retin Eye Res. 2018;62:38–57. https://doi.org/10.1016/j.preteyeres.2017.09.001. Epub 2017/09/21PubMed PMID: 28928087; PMCID: PMC5776047
Fernandez-Godino R, Garland DL, Pierce EA. A local complement response by RPE causes early-stage macular degeneration. Hum Mol Genet. 2015;24:5555–69. https://doi.org/10.1093/hmg/ddv287
Takabayashi T, Vannier E, Clark BD, Margolis NH, Dinarello CA, Burke JF, et al. A new biologic role for C3a and C3a desArg: regulation of TNF-alpha and IL-1 beta synthesis. J Immunol. 1996;156:3455–60. Epub 1996/05/01. PubMed PMID: 8617973
Fernandez-Godino R, Pierce EA. C3a triggers formation of sub-retinal pigment epithelium deposits via the ubiquitin proteasome pathway. Sci Rep. 2018;8:9679 https://doi.org/10.1038/s41598-018-28143-0
Fernandez-Godino R, Bujakowska KM, Pierce EA. Changes in extracellular matrix cause RPE cells to make basal deposits and activate the alternative complement pathway. Hum Mol Genet. 2018;27:147–59. https://doi.org/10.1093/hmg/ddx392
Yamada Y, Ishibashi K, Ishibashi K, Bhutto IA, Tian J, Lutty GA, et al. The expression of advanced glycation endproduct receptors in rpe cells associated with basal deposits in human maculas. Exp Eye Res. 2006;82:840–8. https://doi.org/10.1016/j.exer.2005.10.005
Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci. 2006;103:2328–33. https://doi.org/10.1073/pnas.0408835103
Funding
This work was funded by F. Hoffmann-La Roche, Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
FMD, GW, GD-N, PP, AJ and SF are employees or contractors of F.Hoffmann-La Roche AG.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Altay, L., Sitnilska, V., Schick, T. et al. Early local activation of complement in aqueous humour of patients with age-related macular degeneration. Eye 33, 1859–1864 (2019). https://doi.org/10.1038/s41433-019-0501-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-019-0501-4
This article is cited by
-
Electrochemical nano‐biosensor based on electrospun indium zinc oxide nanofibers for the determination of complement component 3 protein
Microchimica Acta (2023)
-
Epithelial phenotype restoring drugs suppress macular degeneration phenotypes in an iPSC model
Nature Communications (2021)
-
Association of imaging biomarkers and local activation of complement in aqueous humor of patients with early forms of age-related macular degeneration
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)