Abstract
Purpose
To determine presenting visual acuity levels and explore the factors associated with failing vision screening in a multi-ethnic population of UK children aged 4–5 years.
Methods
Visual acuity (VA) using the logMAR Crowded Test was measured in 16,541 children in a population-based vision screening programme. Referral for cycloplegic examination was based on national recommendations (>0.20logMAR in one or both eyes). Presenting visual impairment (PVI) was defined as VA >0.3logMAR in the better eye. Multivariable logistic regression was used to assess the association of ethnicity, maternal, and early-life factors with failing vision screening and PVI in participants of the Born in Bradford birth cohort.
Results
In total, 2467/16,541 (15%) failed vision screening, 732 (4.4%) had PVI. Children of Pakistani (OR: 2.49; 95% CI: 1.74–3.60) and other ethnicities (OR: 2.00; 95% CI: 1.28–3.12) showed increased odds of PVI compared to white children. Children born to older mothers (OR: 1.63; 95% CI: 1.19–2.24) and of low birth weight (OR: 1.52; 95% CI: 1.00–2.34) also showed increased odds. Follow-up results were available for 1068 (43.3%) children, 993 (93%) were true positives; 932 (94%) of these had significant refractive error. Astigmatism (>1DC) (44%) was more common in children of Pakistani ethnicity and hypermetropia (>3.0DS) (27%) in white children (Fisher’s exact, p < 0.001).
Conclusions
A high prevalence of PVI is reported. Failing vision screening and PVI were highly associated with ethnicity. The positive predictive value of the vision screening programme was good, with only 7% of children followed up confirmed as false positives.
Similar content being viewed by others
Introduction
The United Kingdom National Screening Committee (UK NSC) recommends vision screening for all children at aged 4–5 years [1]. This is the first vision test for the majority of children in the United Kingdom and is the key assessment for identifying decreased visual acuity (VA).
A reduction in VA is highly indicative of the presence of an associated condition such as refractive error, strabismus, and/or amblyopia. The UK NSC recommends that all children should have VA measured monocularly and that children failing to achieve ≤0.2logMAR in both eyes should be referred for follow-up testing [1].
World-wide population-based studies have reported a prevalence of presenting visual impairment (PVI, defined as VA of >0.30 logMAR, in the better eye, using spectacles if worn) in children between 0.9 and 1.8% [2,3,4]. The factors associated with reduced VA are known to vary between populations [2, 3, 5,6,7] with the prevalence of refractive error differing between ethnic groups; for example, a higher prevalence of hypermetropia and myopia has been reported in white [8] and East-Asian [9] populations, respectively. The prevalence of strabismus has been reported to vary between 1 [10] and 3% [11], and both the prevalence and type of strabismus has been shown to differ between ethnic groups, with esotropia being more common in children of white ethnicity [12, 13] and exotropia more common in African-American [11] and East-Asian populations [10].
The 2011 census indicated that 6% of the UK population was of South Asian origin; this is the fastest growing ethnic group in the United Kingdom [14]. Ethnicity along with other factors such as socio-economic status, maternal lifestyle choices and prematurity are risk factors associated with amblyopia [7], strabismus [15] and other ophthalmic conditions [16] with the potential to affect visual development.
Population-specific prevalence data are required to inform service provision and knowledge of the risk factors associated with decreased VA in children will inform our understanding of causes and potentially modifiable factors. The aim of this study is to report the VA levels at the point of screening found in a UK multi-ethnic population using the VA referral criteria recommended by the UK NSC [1] (>0.20logMAR in one or both eyes) and explore maternal and early-life factors associated with failing vision screening. A secondary aim is to report on the prevalence of presenting visual impairment (PVI, VA of >0.30 logMAR in the better eye) [2, 4] in the population and again to examine the associated factors.
Methods
Study population
The population-based, vision screening programme in the city of Bradford, UK is offered annually to children commencing school aged 4–5 years. The programme achieves 97% coverage of the target population [17]. Screening is conducted in primary schools by orthoptists and includes VA measurement, cover test and non-cycloplegic auto-refraction (Welch-Allyn Inc Skaneateles, New York, USA). VA is tested monocularly at 3 m (with spectacles if worn) using the logMAR Crowded test (Keeler, Windsor, UK) with a letter matching card and is measured to threshold. For the purposes of this study, the results from all children failing to achieve the VA pass criterion set by the UK NSC [1] (≤0.2logMAR in both eyes) were examined. As per the local protocol, children who failed vision screening but with VA <0.70logMAR were referred for follow-up to a community optometrist of their choice. Those with ≥0.70logMAR were referred to the hospital eye service (HES). All the results from the vision screening programme were recorded and maintained on a secure server in the HES.
Children failing the VA criterion at vision screening were referred initially for a cycloplegic refraction (1% cyclopentolate) and fundus examination undertaken either by a paediatric ophthalmologist or an optometrist, who based on the cycloplegic refraction result, determined whether spectacles were necessary, and if so, what the spectacle prescription should be. Children attending the HES had a follow-up appointment arranged with the orthoptist ~8 weeks after the cycloplegic examination to repeat the VA measurement, wearing any prescribed spectacles. Children assessed by a community optometrist had their examination results returned to the HES and also had a follow-up appointment arranged with the orthoptist. All VA testing, both at the point of vision screening and at follow-up, was performed using the same method of measurement described above. The follow-up results including cycloplegic refraction, VA with the prescribed spectacles, cover testing and fundus, and media examination were extracted from the medical notes following repeated testing. The programme data were collected over a 3-year period between 2012 and 2015.
Bradford is home to the Born in Bradford (BiB) birth cohort, following children born between 2007 and 2011. Details of recruitment have been published previously [18]. In order to explore potential risk factors for failing vision screening and PVI, the vision screening data were linked to data collected from the subset of mothers and children participating in BiB. For each child in the BiB cohort, data on gender, ethnicity, early life [7] (gestational age, route of birth, birth weight) and maternal factors [5] (age, education, smoking in pregnancy and whether receiving state benefits) were linked to the vision screening data. Ethics approval was obtained from the National Research Ethics Committee Yorkshire and the Humber- South Yorkshire UK (Ref. 13/YH/0379) and the study was conducted according to the tenets of the Declaration of Helsinki.
Definitions
Presenting visual acuity (PVA) is the VA of the better eye with spectacles, if worn. Presenting visual impairment (PVI) is defined as VA of >0.3logMAR in the better eye with spectacles if worn [2, 4]. Strabismus was diagnosed at follow-up from cover testing (with and without any prescribed correction) and defined as any manifest deviation (constant or intermittent) at near (33 cm) or distance (6 M). A true positive is defined as VA, at the follow-up appointment with an orthoptist, in the right or left eyes of >0.2logMAR and/or the presence of a significant refractive error confirmed on cycloplegic refraction and/or the presence of an associated ocular factor, e.g., strabismus or ocular motility disorder. A false positive is defined as the absence of a significant refractive error, no associated ocular factor and VA of ≤0.2logMAR in the right and left eyes at follow-up. Based on the result of the cycloplegic refraction, refractive error was defined as follows [19]: low hypermetropia ≥+2.0D to +3.0D spherical equivalent refraction (SER) (sphere plus half cylinder), hypermetropia >+3.0D SER and myopia ≤−0.50D SER. Astigmatism is diagnosed when the cylindrical component of the refractive error was ≥1.0D and emmetropia was defined as >−0.5D to <+2.0D SER in the absence of astigmatism. Failed to attend includes those children who were confirmed to have missed an appointment and also those children for whom there was no confirmatory record, either as the notes were unavailable or there was no confirmation in the notes.
Statistical analysis
Data are presented for all children participating in the annual vision screening programme between 2012 and 2015 in whom VA measures exist for both eyes. A description of the characteristics of the subset of children participating in BiB, including the distribution of early life, maternal risk factors and the VA is detailed. Univariable and multivariable logistic regression was used to further examine the associations between potential risk factors first using the pass/fail criterion for vision screening [1] and second for the criterion for PVI [2, 4]. The factors selected were determined by previously reported literature and include maternal factors (ethnicity, age, level of education, in receipt of UK mean-tested benefits, smoked during pregnancy) and child factors (gender, route of birth, gestational age and low birth weight). Missing risk factor data were imputed using multiple imputation with chained equations [20] using 20 imputed data sets. A sensitivity analysis was performed on complete case, and the results showed similar patterns. Odds ratios (OR) and 95% confidence intervals (CI) are presented.
In order to estimate possible bias from loss to follow-up, the characteristics of BiB children who failed vision screening and subsequently attended for follow-up examination were compared with BiB children who were referred but who failed to attend, using either χ2 (categorical data) or t-tests (continuous data), respectively. The children who failed screening and who attended for follow-up were further categorised as true positives or false positives. The distribution of refractive error categories was examined for all children attending follow-up and then compared by ethnic group using Fisher’s exact tests for the BiB subgroup of children. All statistical analyses were carried out using STATA/SE software (Stata/SE 13 Windows, StataCorp LP, College Station, TX, USA).
Results
Vision screening
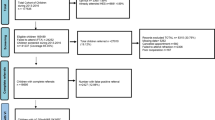
A total of 18,332 children were eligible for screening over the study period and 17,021 completed the screening (Fig. 1). Of these, 380 children were unable to perform the letter matching test and 100 children had VA recorded for only one eye and were thus excluded from the analysis. The remaining 16,541 children had a mean age at the time of testing of 60.07 (SD 4.55) months. Overall, 14,074 (85.1%) children achieved VA ≤0.20logMAR in the both eyes, and so 2467 (14.9%) were referred for follow-up, of these, 775 were BiB children. In total, 732 of the 16,541 children (4.4%) had PVI (VA >0.30 logMAR in the better eye) (Table 1). The mean VA of the right eye (RE) was 0.166 (SD 0.12) logMAR, and the left eye (LE) VA was 0.160 (SD 0.12) logMAR. In total, 354/16,541 (2.1%) children were wearing glasses at the time of vision screening, and of these 136/354 did not pass the screening. No difference was found in age at the time of testing between the children who passed vs those who failed the vision screening (mean diff.: −0.089 months; 95% CI: −0.28 to 0.10, p = 0.35).
Participants' flow chart describing the pathway and visual acuity levels of children participating in the Bradford vision screening programme. BiB born in Bradford cohort study participants, VA visual acuity, RE right eye, LE left eye, FTA child was confirmed to fail to attend an appointment, no confirmed record of attendance = No medical notes were available or no appointment date confirmed within the medical notes. *Visual acuity was retested with glasses where worn
Risk factor analyses
Of the 16,541 children screened, 5276 (31.8%) were BiB participants and thus had risk factor data available (Table 2). Table 3 shows the multivariable logistic regression analyses for the risk factors for failing vision screening and also having decreased PVI. The odds of failing vision screening based on the recommended pass/fail VA criteria increased in children of Pakistani origin (OR: 1.83; 95% CI: 1.42–2.37) compared to white children. Children of low birth weight, children born to older mothers (Table 3) and children in families receiving benefits were also more likely to fail vision screening. A similar pattern was observed for the multivariable analysis exploring factors associated with PVI. Compared to white children, being of Pakistani origin (OR: 2.49; 95% CI: 1.74–3.60) or of other ethnicity (OR: 2.00; 95% CI: 1.28–3.12) increased the odds of PVI. The factors significantly associated with failing vision screening were also associated with presence/absence of PVI with the exception of being a child in a family in receipt of benefits (Table 3).
Follow-up
Of the 2467 children referred for follow-up, no difference was found in the baseline PVA between those who attended follow-up compared to those who did not attend for follow-up (mean diff.: −0.007; 95% CI: −0.02 to 0.007, p = 0.36). In addition, comparison of the demographic and socio-economic factors, in particular ethnicity, of the BiB children who attended follow-up and those who failed to attend was similar (Supplementary Information (SI)).
The average time between screening and the follow-up appointment with spectacles was 23 (SD 18.38) weeks. In total, 1068/2467 (43.3%) attended for follow-up, had their VA measured and had data available for both vision screening and the follow-up examinations, of these 457 were BiB children (Fig. 1). In total, 993/1068 (92.8%) children were true positives. In total, 932/1068 (87.3%) children had the presence of significant refractive error confirmed and had been prescribed glasses (Fig. 1). In total, 92/1068 (8.6%) children followed up had no significant refractive error; of these, 17 had no associated condition, 15 had VA >0.2 in one eye and two had VA >0.2 in both eyes and were referred for additional testing, e.g., electro-diagnostics. The remaining 75 emmetropic children (7% of the 1068 who attended after failing screening) were found at follow-up examination to have VA of ≤0.2logMAR in both eyes and to be without any significant refractive error or other associated condition. These children were classed as false positives; therefore, 93% of those who failed vision screening were true positives.
In total, 351/457 (76.8%) of the BiB children who attended follow-up were found to have a significant refractive error. Of these, 133 (76.1%) had astigmatism which was the most frequent refractive error type (Table 4). Astigmatism alone or in combination with myopia was more frequent in the children of Pakistani origin compared to white children (Fisher’s exact test, p < 0.001). Both low hypermetropia and hypermetropia were more common in white children, with other ethnicities occupying a middle position in both (Fisher’s exact test, p < 0.001).
Discussion
This study presents a detailed profile of VA measured at vision screening in children aged 4–5 years. Linkage of the screening data with maternal and early-life data from the BiB birth cohort has allowed examination of factors associated with failing vision screening and those associated with PVI. It is one of very few cohort studies reporting a population of South Asian (mainly, Pakistani origin) children. The yield from the screening was high, with 14.9% of the children failing to meet the UK NSC VA pass criteria [1] and 4% having PVI. The vision screening programme showed good positive predictive value (93%) with a false positive rate of only 7%, well within an acceptable standard [21].
Our analyses show that ethnicity, mother’s age at pregnancy and low birth weight are associated with both failing vision screening and PVI. Other population-based studies have reported factors such as ethnicity, gestational age, birth weight, the level of mother’s education and her lifestyle choices to be associated with a reduction in VA [5], strabismus [15] and amblyopia [7, 19]. Also, an Australian cohort study found an association between lower normative VA (VA levels in children without refractive error or ocular disease) and prematurity [22].
The Bradford population is largely bi-ethnic with a high degree of homogeneity for both the Pakistani and white children, as well as having a small but significant proportion of children of other ethnicities [18] (Table 2). This has allowed robust and detailed analysis of the association of ethnicity with PVA in our population. We found that being a child of Pakistani origin had a strong association with failing vision screening (OR: 1.83; 95% CI: 1.42–2.37) and PVI (OR: 2.49; 95% CI: 1.74–3.60). In the United Kingdom, two studies in predominantly white populations report 0.6% [7] and 1.5% [4] of 7-year-old children with PVI. In a study in urban New Delhi [23], 4.9% (comparable to our population) of South Asian children were found to have PVI; this differs from rural South India [24] where 2.6% of children were reported to have PVI. The difference between the New Delhi and Southern India studies may be due to differences in the age of children, with those aged 5–7 years excluded from the latter study due to inability to perform the vision test. Leone et al. [22], reporting normative VA in preschool children, found East-Asian children to have a lower mean VA compared to European or South Asian children of the same age and Merritt et al. [25] report higher prevalence of decreased VA among African Americans (8.4%) compared to white American (4%) preschool children. However, a number of studies reporting both normative VA [26] and decreased VA [2, 3] in different populations have found no significant ethnic differences.
Socio-economic factors have also been reported to be associated with VA. In a Scottish study children from the most deprived backgrounds were highly likely to fail vision screening compared to those from the least deprived backgrounds (OR: 3.59, 95% CI: 1.6–7.8, p = 0.001) [27] and in the United States a study reported the socio-economic markers of lack of health insurance and lower educated mothers to be associated with bilateral decreased VA in preschool children [3]. We found being in receipt of means tested benefits was associated with failing vision screening but not with PVI, possibly because of lower statistical power given the smaller number of children with PVI.
All children failing to meet the UK NSC pass criterion [1] were referred however, a significant number failed to attend (Fig. 1). No socio-economic or demographic difference was found between the BiB children that failed to attend compared to those that attended follow-up (Supplementary Information). This may be due to the relative deprivation [28] within the local population [18]. Of those children who attended follow-up a large majority (87%) were found, following cycloplegic refraction, to require spectacles. This supports the case for all children failing vision screening to have a cycloplegic refraction to identify refractive error performed as part of the follow-up pathway. Our findings are similar to those of previous studies in which the presence of significant refractive error was found to be highly associated with reduced VA in young children [2, 5, 6, 27]. We found that astigmatism alone or in combination with spherical ametropia was more common in children of Pakistani ethnicity (Fisher’s exact, p < 0.001), and hypermetropia was more common in white children (Fisher’s exact, p < 0.001). In the United Kingdom, Fuller et al., studying a small sample of 62 children, reported higher prevalence (22.6%) of astigmatism in children of Bangladeshi origin compared to white children aged 4–5 years in two London schools [29]. The association of astigmatism with reduced VA has been reported in the combined findings of two large population-based studies in the United States [5], in which the odds of reduced VA were positively associated with the presence of astigmatism of >2.0D (OR: 17.6; 95% CI: 9–34.5). An Australian study of children of mainly white ethnicity has also reported astigmatism (≥1.0DC) as the principal refractive error leading to reduced VA [2].
Similarly, the prevalence of hypermetropia is reported to vary between populations and between ethnic groups [30]. In the United Kingdom, a study of white children in Northern Ireland reported a 26% [4] prevalence of hypermetropia (≥3.00D) at age 6–7 years while a large cohort study in Bristol, UK reported a prevalence of just 5% [7] in children of mainly white ethnicity at the age of 7 years. The difference between the studies is likely to be due, at least in part, to the lack of cycloplegic refraction in the Bristol study. An Australian study [31] of 6-year-old children, using cycloplegic auto-refraction in a multi-ethnic population, reported 13.2% prevalence of hypermetropia (≥3.0D) in their population, with white children (15.7%) having an increased prevalence compared to children of other ethnicities (6.8%). An American study [8], also using cycloplegic auto-refraction, reported 8.9% of white children and 4.4% of African-American children to have hypermetropia (>3.0D).
We collected population-based screening data annually between 2012 and 2015. This large population base allows the presentation of VA levels with exploration and detailed analysis of associated risk factors for both failing vision screening and PVI. Both the initial VA measures at screening and the repeat VA measure at follow-up were collected by orthoptists with a high level of training and experience in VA measurement in young children, thus providing consistency of testing. However, this study has limitations. This paper presents data collected from clinical practice based on follow-up of 43.3% of the children referred, due to a combination of confirmed non-attendance and an inability to locate examination notes or to confirm attendance in medical notes. There is potential bias, particularly if one ethnic group was less likely to attend or if the level of PVA differed between attenders and non-attenders. However, no significant difference was found in the PVA (mean diff.: −0.007; 95% CI: −0.02 to 0.007) between children who attended and those who failed to attend, nor was a difference found for any demographic or socio-economic characteristic between BiB children who attended and those who failed to attend (SI). On this basis, the VA levels and the relative frequency of refractive errors reported in the different ethnic groups of children who attended follow-up is likely to be representative of all children who failed screening.
The cycloplegic examination was undertaken by ophthalmologists or optometrists either in the community, or in the HES. The fact that cycloplegic refraction was conducted by a wide range of eye care professionals means that the examinations were not standardised, nor was there standardisation of the adjustment (if any) to the cycloplegic result in relation to what was prescribed. However, this reflects clinical practice in the United Kingdom.
Children who passed the screening were not followed up preventing the identification of children who may actually have had a reduction in VA, thus we are not able to identify the proportion of false negatives in our sample.
An understanding of the prevalence, epidemiology and natural history of the target condition(s) is required to inform guidance and recommendations for national screening programmes. Identification of reduced VA is important in young children as it allows early detection and treatment of the related childhood eye disorders. Our study adds to current knowledge by providing robust prevalence data and valuable evidence of maternal and early life risk factors for failing vision screening and exhibiting PVI, highlighting the importance of the demographic profile of the target population. The high prevalence (4.4%) of PVI has implications for the planning and provision of vision screening programmes and the subsequent referral pathways to ophthalmological, orthoptic and optometry care. This study provides an epidemiological benchmark for similar urban populations and presents policy makers with information which will help in the planning of such services.
Summary
What was known before
-
In the United Kingdom, children from predominantly white populations are reported to have a low prevalence (0.6–1.5%) of presenting visual impairment (defined as logMAR >0.3 in the better eye).
-
World-wide, there is evidence for an association between ethnicity and presenting visual impairment.
-
Socio-economic factors have also been reported to be associated with presenting visual impairment.
What this study adds
-
A prevalence of presenting visual impairment of 4.4% was found at vision screening in this UK multi-ethnic population at age 4–5 years.
-
Robust evidence that children of Pakistani origin have around twice the odds of both failing vision screening and having presenting visual impairment, compared to white British children.
-
The positive predictive value of the vision screening programme based on UK National Screening Committee (UK NSC) recommendations was high (93%) with only 7% of children followed up confirmed as false positives.
References
National Screening Committee. The UK NSC policy on vision defects screening in children 2013. Available at http://www.screening.nhs.uk/vision-child. Accessed 13 June 2017.
Robaei D, Rose K, Ojaimi E, et al. Visual acuity and the causes of visual loss in a population-based sample of 6-year-old Australian children. Ophthalmology. 2005;112:1275–82.
Friedman DS, Repka MX, Katz J, et al. Prevalence of decreased visual acuity among preschool-aged children in an American urban population: the Baltimore Pediatric Eye Disease Study, Methods, and Results. Ophthalmology. 2008;115:1786–95.
O’Donoghue L, McClelland JF, Logan NS, et al. Refractive error and visual impairment in school children in Northern Ireland. Br J Ophthalmol. 2010;94:1155–59.
Tarczy-Hornoch K, Varma R, Cotter SA, Joint Writing Committee for the Multi-Ethnic Pediatric Eye Disease Study the Baltimore Pediatric Eye Disease Study Groups. et al. Risk factors for decreased visual acuity in preschool children: the multi-ethnic pediatric eye disease and Baltimore Pediatric Eye Disease Studies. Ophthalmology. 2011;118:2262–73.
Pan C-W, Chen X, Gong Y, et al. Prevalence and causes of reduced visual acuity among children aged three to six years in a metropolis in China. Ophthalmic Physiol Opt. 2016;36:152–57.
Williams C, Northstone K, Howard M, et al. Prevalence and risk factors for common vision problems in children: data from the ALSPAC study. Br J Ophthalmol. 2008;92:959–64.
Giordano L, Friedman DS, Repka MX, et al. Prevalence of refractive error among preschool children in an urban population: the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116:739–46. 46 e1-4
Dirani M, Chan YH, Gazzard G, et al. Prevalence of refractive error in Singaporean Chinese children: the strabismus, amblyopia, and refractive error in young Singaporean Children (STARS) study. Invest Ophthalmol Vis Sci. 2010;51:1348–55.
Matsuo T, Matsuo C. The prevalence of strabismus and amblyopia in Japanese elementary school children. Ophthal Epidemiol. 2005;12:31–36.
Friedman DS, Repka MX, Katz J, et al. Prevalence of amblyopia and strabismus in White and African American children aged 6 through 71 months: the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116:2128–34.e2.
Bruce A, Santorelli G. Prevalence and risk factors of strabismus in a UK multi-ethnic birth cohort. Strabismus. 2016;24:153–60.
Cotter SA, Varma R, Tarczy-Hornoch K, et al. Risk factors associated with childhood strabismus: the multi-ethnic pediatric eye disease and Baltimore pediatric eye disease studies. Ophthalmology. 2011;118:2251–61.
Office for National Statistics. Census: aggregate data (England and Wales) [computer file]. UK Data Service Census Support. Available at http://infuse.ukdataservice.ac.uk. This information is licensed under the terms of the Open Government Licence [http://www.nationalarchives.gov.uk/doc/open-government-licence/version/2]. 2011.
Pathai S, Cumberland PM, Rahi JS. Prevalence of and early-life influences on childhood strabismus: findings from the Millennium Cohort Study. Arch Pediatr Adolesc Med. 2010;164:250–57.
Cumberland PM, Pathai S, Rahi JS. Prevalence of eye disease in early childhood and associated factors: findings from the millennium cohort study. Ophthalmology. 2010;117:2184–90 e1-3.
Bruce A, Outhwaite L. Uptake, referral and attendance: results from an inner city school based vision screening programme. Br Ir Orthopt J. 2013;10:41–45.
Wright J, Small N, Raynor P, et al. Cohort profile: the born in Bradford multi-ethnic family cohort study. Int J Epidemiol. 2013;42:978–91.
Robaei D, Rose KA, Ojaimi E, et al. Causes and associations of amblyopia in a population-based sample of 6-year-old Australian children. Arch Ophthalmol. 2006;124:878–84.
White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–99.
Public Health England. Child vision screening resources consultation. Available at https://www.gov.uk/government/consultations/child-vision-screening-resources. 2017. Accessed 04 August 2017.
Leone JF, Mitchell P, Kifley A, et al. Normative visual acuity in infants and preschool-aged children in Sydney. Acta Ophthalmol. 2014;92:e521–e29.
Murthy GVS, Gupta SK, Ellwein LB, et al. Refractive error in children in an urban population in New Delhi. Invest Ophthalmol Vis Sci. 2002;43:623–31.
Dandona R, Dandona L, Srinivas M, et al. Refractive error in children in a rural population in India. Invest Ophthalmol Vis Sci. 2002;43:615–22.
Merritt JC, Game S, Williams OD, et al. Visual acuity in preschool children: the Chapel Hill-Durham Day-Care Vision Study. J Natl Med Assoc. 1996;88:709–12.
Pan Y, Tarczy-Hornoch K, Susan AC, et al. Visual acuity norms in preschool children: the Multi-Ethnic Pediatric Eye Disease Study. Optom Vis Sci. 2009;86:607–12.
O’Colmain U, Low L, Gilmour C, et al. Vision screening in children: a retrospective study of social and demographic factors with regards to visual outcomes. Br J Ophthalmol. 2016;100:1109–13.
Sharp DJ, Hamilton W. Non-attendance at general practices and outpatient clinics: local systems are needed to address local problems. BMJ. 2001;323:1081–82.
Fuller JR, Baxter LA, Harun S, et al. Astigmatism in Bangladeshi and white school entrants in East London: a prospective comparative study. Eye. 1995;9:794–96.
Ying GS, Maguire MG, Cyert LA, et al. Prevalence of vision disorders by racial and ethnic group among children participating in head start. Ophthalmology. 2014;121:630–6.
Ip JM, Robaei D, Kifley A, et al. Prevalence of hyperopia and associations with eye findings in 6- and 12-year-olds. Ophthalmology. 2008;115:678–85 e1.
Acknowledgements
The authors thank all the families and schools who took part in this study, the orthoptists from Bradford Teaching Hospitals Foundation Trust who conducted the vision screening programme and the Data Support Team from Bradford Institute for Health Research who created and maintained the data linkage system.
Funding
AB is funded by a National Institute for Health Research Post-Doctoral Fellowship Award (PDF-2013-06-050). The Born in Bradford study presents independent research commissioned by the National Institute for Health Research Collaboration for Applied Health Research and Care (NIHR CLAHRC) and the Programme Grants for Applied Research funding scheme (RP-PG-0407-10044). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author contributions
AB initiated the project, designed data collection, monitored data collection for the whole study, wrote the statistical analysis plan, cleaned and analysed the data, and drafted and revised the paper. She is the guarantor. GS wrote the statistical analysis plan, cleaned and analysed the data and revised the draft paper. JAB, BTB and MB contributed to the design of the study and revised the draft paper. TAS and JW initiated the project, contributed to the design of the study and revised the draft paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bruce, A., Santorelli, G., Wright, J. et al. Prevalence of, and risk factors for, presenting visual impairment: findings from a vision screening programme based on UK NSC guidance in a multi-ethnic population. Eye 32, 1599–1607 (2018). https://doi.org/10.1038/s41433-018-0146-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-018-0146-8