Abstract
Purpose
This study is aimed to adapt a three-dimensional (3-D) in vitro angiogenesis model to the ophthalmology field using retinal endothelial cells (REC). This system is applied to assess the angiogenic capacity of aqueous humor (AH) from patients with ocular disorders, and to test the effect of VEGF inhibitor (aflibercept) on induced angiogenesis.
Methods
Human REC and umbilical vein endothelial cells (HUVEC) and pericytes were co-cultured in a gel matrix with 25–200 ng/ml pro-angiogenic growth factors (GF). AH from patients with cataract, glaucoma or proliferative diabetic retinopathy (PDR) was tested in the REC-pericyte co-culture. Aflibercept was then introduced to the co-culture containing PDR AH. The surface area and total tubule length were measured using Image J.
Results
Optimal GF concentrations at 200 ng/ml induced angiogenesis by REC as well as HUVEC, while vessel formation by both cell types was strongly reduced using 25–50 ng/ml GF. Addition of AH from the PDR patient triggered tubule formation by REC at low GF concentration. Aflibercept, however, significantly inhibited angiogenesis induced by PDR AH, but showed no significant influence on other conditions.
Conclusion
REC can be applied efficiently in the 3-D in vitro angiogenesis model as a diagnostic tool to assess the AH angiogenic status and to validate new anti-angiogenic therapeutic compounds prior to clinical trial.
Similar content being viewed by others
Introduction
Retinal neovascularization at the vitreoretinal interface is associated with several common eye disorders, which may lead to permanent blindness. These include proliferative diabetic retinopathy (PDR), retinopathy of prematurity, retinal vein occlusions and sporadic cases of uveitis. Several pathological processes may influence the retinal vasculature and result in vessel obstruction, hypoxia, and subsequent ischemia followed by retinal neovascularization [1, 2]. Although neovascularization in principle occurs as a compensatory repair process, the newly formed vessels contain multiple local structural and functional defects. Such abnormalities make these vessels fragile and prone to rupture, causing intraocular hemorrhage and subsequent fibrosis with retinal detachment, which is a major cause of vision loss.
Neovascularization is a complex process and involves several variables, including vascular and mural cells as well as immune cells, different cytokines/growth factors and extracellular matrix components (recently reviewed in [3]). Vascular endothelial growth factor (VEGF) is a major pro-angiogenic factor and its uncontrolled activity is highly associated with pathological retinal angiogenesis [1]. Therefore, VEGF is currently the treatment target of choice to inhibit pathological ocular angiogenesis.
Clinically relevant neovascularization models are essential to improve our understanding of biological mechanisms underlying retinal neovascularization, and to increase insight into the potential efficacy of a drug, a disease entity or even a specific patient [3, 4].
In the current study, we apply a three-dimensional (3-D) in vitro angiogenesis assay using human retinal endothelial cells (REC) and pericytes for the use in ophthalmology. We evaluated its potential application by 1) examining the effect of aqueous humor (AH) from a patient with PDR, a patient with glaucoma and a patient with cataract, and 2) examining the inhibitory potential of aflibercept (Eylea; VEGF-trap) on AH-induced vessel formation.
Materials and methods
Aqueous humor samples
AH samples were obtained from the local biobank (CORRBi: combined ophthalmic research Rotterdam biobank) for which signed informed consent by the patients and approval of the local medical ethics committee was given. The study included an AH sample of a cataract patient, a glaucoma patient, and a type-2 diabetes mellitus patient with PDR.
Cell culture
Human umbilical vein endothelial cells (HUVEC) (Lonza, Verviers, Belgium, cat# CC-2519) and human REC (Cell Systems, Troisdorf, Germany, certificate# ACBRI 181) were transduced with a green fluorescent protein (GFP)-encoding lentiviral vector and cultured subsequently in EGM2 and EGM medium (Lonza), respectively, supplemented with penicillin/streptomycin as previously described [5]. Fetal bovine serum (FBS) was used as 2 and 5% in EGM2 and EGM medium, respectively.
Human brain vascular pericytes (HBVP, ScienCell, San Diego, USA, cat# 1200) were similarly transduced with lentivirus encoding discosoma sp. red fluorescent protein (dsRED) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Lonza) supplemented with penicillin/streptomycin and 10% FBS [5].
All the cell types were tested negative for mycoplasma infection prior to use.
Three-dimensional tubule formation assay
GFP-labeled endothelial cells (EC) (0.3–0.45 × 106) and dsRED-labeled pericytes (6–9 × 104) were suspended in EBM-2 medium (Lonza) containing ascorbic acid, recombinant human fibroblast growth factor (FGF) and 2% FBS from the EGM2 bulletkit (Lonza, cat # 3162) (co-culture medium). The cell suspension was supplemented with growth factors: stem cell factor (SCF), stromal cell-derived factor-1α (SDF-1α, CXCL12), and interleukin-3 (IL-3) (R&D systems, Abingdon, UK; 25–200 ng/ml as indicated), and the volume was adjusted to 300 µl with co-culture medium. Subsequently, bovine collagen type-I (Gibco, Landsmeer, Netherlands) was added to a final concentration of 2.5 mg/ml, and 50 µl of the mixture was loaded per well of a 96-well plate in at least four replicates. The pH of the collagen solution was neutralized to pH 7.0 using 1 M NaOH prior to co-culture. After an hour of co-culture, 100 µl of co-culture medium was gently added on top of the solidified gel to protect the gel from shrinkage and provide sufficient nutrients for the cells [6].
To analyze the angiogenic properties of AH samples from patients, the biomaterials were used in the gel phase in the co-culture system as 5% of the total volume of the mixture. The potential inhibitory effect of the VEGF-neutralizing agent, aflibercept (a recombinant fusion protein consisting of portions of human VEGF receptors 1 and 2 extracellular domains fused to the Fc portion of human IgG1; Regeneron Pharmaceuticals, Tarrytown, NY) on the tubule formation induced by AH samples was then assessed by adding the drug (0.01 µg/ml) to the co-culture.
Cell imaging and image analysis
Tubule formation was monitored at day 5 of co-culture. This time point appeared optimal from initial experiments where tubule formation was determined daily for a period of 5 days (data not shown). An inverted fluorescence microscope (Olympus SC30, Shinjuku, Japan) using ×20 magnification was applied for imaging, and tubule formation was quantified by Image J software (version 1.48) based on surface area and total tubule length percentage. In a script written in Image J (available upon request), particles with a pixel size between “150” and “infinity” were considered as vessels formed by EC in a specific region of interest. The particles were then analyzed based on the surface area percentage in an adjusted threshold. In addition, the structures were skeletonized in binary images, which consist of single-pixel lines, considered as tubules. Accordingly, the percentage of surface area was measured and referred to as total tubule length percentage.
Statistics
The data were analyzed by nonparametric one-way ANOVA test for comparison between control and experimental conditions using Graphpad Prism 5. Unpaired or paired two-tailed student’s t test was applied to determine the statistical difference in tubule formation between each two conditions. A p value < 0.05 was considered to indicate a statistical significant difference. All the data are presented in means ± SD.
Results
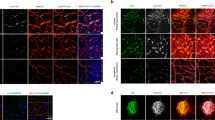
Under co-culture conditions with the pro-angiogenic factors (SCF, CXCL12, IL-3) at a concentration of 200 ng/ml, REC formed tubules as well as HUVEC did (Fig. 1), indicating that REC are capable of interacting optimally with pericytes that guide endothelial sprouting and tubule formation. Remarkably, REC outperformed HUVEC by generating a larger surface area (P = 0.039) and larger tubule length (p = 0.006) as compared to HUVEC. Reduction of the concentration of pro-angiogenic factors to 25 ng/ml led to significant impairment of tubule formation, for both REC and HUVEC (Fig. 1).
REC and HUVEC are capable of sprouting and forming vessels in the three-dimensional in vitro tubule formation assay. a GFP-labeled REC and HUVEC (0.45 × 106 cells) were co-cultured with dsRed-tagged human brain-derived pericytes (9 × 104 cells) in 3-D bovine collagen matrix containing 200 or 25 ng/ml growth factors (GF; SCF, SDF-1α, IL-3). REC formed vessel-like structures in the presence of 200 ng/ml GF, even more efficiently than HUVEC (surface area and tubule length p values < 0.05; see b). In low GF concentration, a significant reduction in in vitro angiogenesis was observed in comparison with the high GF-concentrated co-culture (p < 0.0001; see b). b Quantification of in vitro angiogenesis conditions as shown in (a) represented as total tubule surface area and tubule length of the formed structures. Values show means ± SD from 7 replicates from a representative experiment, which was repeated at least once with similar results. GF growth factors, HUV/ HUVEC human umbilical vein endothelial cells, REC retinal endothelial cells. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001
To investigate a potential stimulatory effect of patient AH samples on REC tubule formation, AH was used in the assay in the presence of pro-angiogenic factors at a concentration of 50 ng/ml. This was compared to the effect of the pro-angiogenic factors (50 and 200 ng/ml) alone. Introduction of 5% cataract AH showed no difference compared to that of 50 ng/ml pro-angiogenic factors alone (Fig. 2). AH from a glaucoma patient showed a slight stimulatory effect on tubule formation by REC, yet this was not statistically significant. In contrast, AH from a PDR patient showed a strong stimulatory effect on REC tubule formation compared to the 50 ng/ml of pro-angiogenic factors alone (surface area p = 0.017, total tubule length p = 0.028), and these reached values comparable to that of cultures stimulated optimally with 200 ng/ml of pro-angiogenic factors.
Aqueous humor from patients with ocular diseases differentially stimulates in vitro retinal angiogenesis. a GFP-tagged REC (0.3 × 106 cells) and dsRed-labeled pericytes (6 × 104 cells) were co-cultured in 3-D bovine collagen gel containing 200 and 50 ng/ml GF. In low GF concentration, tubule formation by REC was significantly reduced compared to the optimal stimulation with 200 ng/ml GF (p < 0.05; see b). Aqueous humor (AH) from cataract and glaucoma patients had no effect on tubule formation, while AH from a proliferative diabetic retinopathy (PDR) patient stimulated tubule formation by REC in low GF concentration (p < 0.05; see b). b Quantification of in vitro angiogenesis conditions as shown in a represented as total tubule surface area and tubule length of the formed structures. Values show means ± SD from 4 replicates from a representative experiment, which was repeated at least once with similar results. AH aqueous humor, PDR proliferative diabetic retinopathy * p < 0.05, ** p < 0.01
Subsequently, the effect of aflibercept on AH-induced tubule formation was examined (Fig. 3). Aflibercept (0.01 μg/ml) significantly inhibited PDR AH-induced tubule formation (tubule length p = 0.049, surface area not significant) to the level of that of 50 ng/ml of pro-angiogenic factors alone. Aflibercept did not affect optimally-dosed co-cultures, nor did we find differences in glaucoma- or cataract patient AH-stimulated cultures in the presence or absence of the inhibitor (data not shown).
REC in vitro angiogenesis, stimulated by aqueous humor from a patient with diabetic retinopathy, is blocked by aflibercept. a Representative images of REC in co-culture assay stimulated with different concentrations of GF as indicated and treated with PDR AH in the presence and absence of VEGF inhibitor aflibercept. Aflibercept blocked REC tubule formation induced by PDR AH (p < 0.05; see b), but had no effect on control co-culture with 50 ng/ml GFs. b Quantification of in vitro angiogenesis conditions as shown in a represented as total tubule surface area and tubule length of the formed structures. Values show means ± SD from 4 replicates from a representative experiment, which was repeated at least once with similar results. Statistical analysis has been performed only on the relevant conditions while the other conditions are included as technical controls. Inh inhibitor aflibercept * p < 0.05
Discussion
In the current study, we have adapted a 3-D in vitro angiogenesis model for application in ophthalmology research and diagnostics. We show that REC are capable to interact optimally with pericytes to sprout and form tubules, at levels at least comparable to those by macrovascular HUVEC. Using suboptimal growth factor concentrations, we have observed in our assay differential stimulatory effects of AH from patients with ocular diseases, as well as effective reduction of induced angiogenesis by the pharmacological inhibitor aflibercept. The major vasoproliferative effect was found with AH from patient with PDR.
Our model is an adaptation of the model created by Stratman et al. [7], which appears to be the one best reflecting the in vivo situation. In their model, which is considered the gold standard in the fields of oncology and cardiology, HUVEC and pericytes are co-cultured in low-serum culture medium containing collagen type-I, FGF and other defined angiogenic growth factors [6,7,8,9]. After 72–120 h of culture, the formation of vessel-like tubules from the sprouting EC can be quantified. We modified this in vitro 3-D angiogenesis model by using REC and optimizing the culture conditions. Using an in vitro system for angiogenesis has several advantages over in vivo animal models. An in vitro model provides an opportunity to carry out human-oriented studies, to evaluate the effect of different stimulatory and inhibitory factors in the absence of other interfering pathological agents, and to test new potential therapeutics. In addition, it allows estimating the efficient dose of drugs, while it is less complicated, more easily reproducible, less time-consuming and less expensive than animal-based studies. Despite its advantages, an in vitro assay also has its limitations, in particular, since the influence of the in vivo microenvironment in healthy or diseased conditions is lost [10, 11].
To date, several studies have reported in vitro angiogenesis assays for rapid screening of potential anti-angiogenic compounds in the field of ophthalmology [11]. Frequently, 2-D tubule formation assays are used by applying EC only [11]. The limitation of a 2-D angiogenesis model is that cell–cell and cell–matrix interactions are not optimal or not at all accounted for. Hence, a 3-D assay as applied here is highly desirable.
In most of the reported in vitro studies, HUVEC have been used to evaluate EC proliferation, migration and extracellular matrix invasion [11]. Since EC in different organs and situations show remarkable heterogeneity in morphology and function [12], it is far from ideal to use macrovascular EC such as HUVEC in retinal angiogenesis studies. Some other studies have used immortalized or primary bovine REC for the same purpose in ex vivo or in vitro 2-D assays, since isolation of primary human REC provides only a limited supply of cells [11]. In addition, previous studies have assessed angiogenic capacity of compounds in 3-D assays using murine and bovine retinal explants cultured in collagen gel [13, 14]. Another study conducted by Rezolla and colleagues demonstrated the inhibitory effect of anti-VEGF antibodies on VEGF-induced angiogenesis by mouse primary REC in a 3-D fibrin gel culture [15]. Together, these studies generated important information about retinal vascularization, but it should be realized that microvascular EC from different species show significant variation in gene expression and phenotype [16]. Therefore, we argue that using REC from human origin is essential for experiments aiming at studying the mechanisms of human retinal angiogenesis.
Taken together, we propose that this application can represent a productive experimental model to assess basic mechanisms in retinal angiogenesis as well as to test novel anti-angiogenic therapeutics for retinal microvascular diseases. Our model allows as well determining the resistance to current anti-angiogenic treatment modalities in specific ocular diseases, which might be related to not only the specific stage of retinal disease but also to differential involvement of distinct angiogenic factors. Adaptation of the model by applying choroidal EC would make our method applicable for other common microvascular ocular disorders, including wet age-related macular degeneration (AMD).
Summary
What was known before
-
Patients with proliferative diabetic retinopathy (PDR) show dysfunctional retinal neovascularization.
-
In vitro 3-dimensional (3-D) models of neovascularization have been developed in which human umbilical vein endothelial cells (HUVEC) integrate with pericytes in collagen matrix to form vessel-like structures.
-
Aflibercept binds to VEGF and inhibits VEGF-induced angiogenesis.
What this study adds
-
We developed a physiological model of retinal angiogenesis, in which retinal endothelial cells (REC) replace HUVEC in an in vitro 3-D angiogenesis assay in studies related to microvascular retinal disorders.
-
Aqueous humor (AH) from patients with PDR stimulates angiogenesis in the 3-D angiogenesis using REC.
-
Aflibercept inhibits the in vitro angiogenesis induced by PDR AH.
-
The 3-D in vitro angiogenesis assay using REC can be used as a model to study (pathologic) neovascularization in the field of ophthalmology and the efficacy of medication to retinal neovascularization.
References
Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81.
Bishop PN. The role of extracellular matrix in retinal vascular development and preretinal neovascularization. Exp Eye Res. 2015;133:30–6.
van Beijnum JR, Nowak-Sliwinska P, Huijbers EJ, Thijssen VL, Griffioen AW. The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol Rev. 2015;67:441–61.
Siemerink MJ, Augustin AJ, Schlingemann RO. Mechanisms of ocular angiogenesis and its molecular mediators. Dev Ophthalmol. 2010;46:4–20.
Yan T, Wang M, Xu Z, Huang C, Zhou X, Jiang E, et al. Up-regulation of syncytin-1 contributes to TNF-α-enhanced fusion between OSCC and HUVECs partly via Wnt/β-catenin-dependent pathway. Sci Rep. 2017;7:1–12.
Zhou ZC, Chrifi I, Xu YJ, Pernow J, Duncker DJ, Merkus D, et al. Uridine adenosine tetraphosphate acts as a proangiogenic factor in vitro through purinergic P2Y receptors. Am J Physiol-Heart C. 2016;311:H299–309.
Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–30.
Zhu C, Chrifi I, Mustafa D, van der Weiden M, Leenen PJM, Duncker DJ, et al. CECR1-mediated cross talk between macrophages and vascular mural cells promotes neovascularization in malignant glioma. Oncogene. 2017;36:5356–68.
Chrifi I, Louzao-Martinez L, Brandt M, van Dijk CGM, Burgisser P, Zhu C, et al. CMTM3 (CKLF-like marvel transmembrane domain 3) mediates angiogenesis by regulating cell surface availability of ve-cadherin in endothelial adherens junctions. Arterioscler Thromb Vasc Biol. 2017;37:1098–114.
Ucuzian AA, Greisler HP. In vitro models of angiogenesis. World J Surg. 2007;31:654–63.
Rezzola S, Belleri M, Gariano G, Ribatti D, Costagliola C, Semeraro F, et al. In vitro and ex vivo retina angiogenesis assays. Angiogenesis. 2014;17:429–42.
Stewart EA, Samaranayake GJ, Browning AC, Hopkinson A, Amoaku WM. Comparison of choroidal and retinal endothelial cells: characteristics and response to VEGF isoforms and anti-VEGF treatments. Exp Eye Res. 2011;93:761–6.
Im E, Venkatakrishnan A, Kazlauskas A. Cathepsin B regulates the intrinsic angiogenic threshold of endothelial cells. Mol Biol Cell. 2005;16:3488–500.
Shafiee A, Penn JS, Krutzsch HC, Inman JK, Roberts DD, Blake DA. Inhibition of retinal angiogenesis by peptides derived from thrombospondin-1. Invest Ophthalmol Vis Sci. 2000;41:2378–88.
Rezzola S, Belleri M, Ribatti D, Costagliola C, Presta M, Semeraro F. A novel ex vivo murine retina angiogenesis (EMRA) assay. Exp Eye Res. 2013;112:51–6.
Bharadwaj AS, Appukuttan B, Wilmarth PA, Pan Y, Stempel AJ, Chipps TJ, et al. Role of the retinal vascular endothelial cell in ocular disease. Prog Retin Eye Res. 2013;32:102–80.
Acknowledgements
We thank Dr. O. G. de Jong for donating the lentiviral GFP and dsRED constructs. Research for this manuscript was (in part) performed within the framework of the Erasmus post-graduate school Molecular Medicine and supported by Uitzicht grant (Uitzicht 2015-10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shariatzadeh, M., Brandt, M.M., Cheng, C. et al. Three-dimensional tubule formation assay as therapeutic screening model for ocular microvascular disorders. Eye 32, 1380–1386 (2018). https://doi.org/10.1038/s41433-018-0089-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-018-0089-0