Abstract
Oculofaciocardiodental (OFCD) syndrome is a rare X-linked dominant syndrome characterized by the involvement of the eyes, face, teeth, and heart with variable expressivity. The syndrome is caused by loss-of-function variants in the BCOR gene located on the X chromosome. OFCD affects only females with presumed embryonic lethality among males. We report a first case of a female with biallelic mosaic variants in BCOR gene, leading to a severe ocular phenotype including anterior segment dysgenesis, cataracts, and retinal involvement. The unique condition of biallelic mosaic loss-of-function mutations leads to a variable expression of an allele with the pathogenic variant, independent of the X-Inactivation pattern. This novel mechanism of co-existent biallelic mosaicism should be suspected in unexplained severe cases of OFCD.
Similar content being viewed by others
Introduction
Oculofaciocardiodental(OFCD) Syndrome is a rare genetic disorder affecting females causing microphthalmia, congenital cataracts, radiculomegaly, cardiac and digital abnormalities [1, 2]. The syndrome is inherited by X-linked dominant fashion and is presumed to be lethal in males. The syndrome is caused by null mutations in the BCOR gene (encoding BCL-6-interacting corepressor) [3]. Interestingly, variants in BCOR gene were found to be associated with Lenz microphthalmia, which is inherited by X-linked recessive pattern with an overlapping phenotype [3]. Most cases of OFCD are sporadic and caused by de novo variants, but familial cases inherited from an affected mother have been reported [4]. Suspected germline mosaicism associated with inheritance of heterozygous pathogenic variants have been reported [5]. Moreover, a female diagnosed with isolated ocular defects and identified minor features of OFCD was diagnosed with a mosaic somatic pathogenic variant in BCOR gene [6].
In this case report, we present a rare case of a child with biallelic mosaic variants in BCOR gene and a severe ophthalmic phenotype including bilateral cataract, microphthalmia, iris, and ciliary body abnormalities, as well as retinal involvement.
Subjects and methods
A 6-month-old baby girl was born to non-consanguineous Ashkenazi Jewish parents after uneventful pregnancy. Gestational age at delivery was 38 + 3 weeks with a birth weight of 2500gr. Apgar scores were 9 and 10 after 1 and 5 min, respectively. Due to a systolic murmur, an echocardiogrdiogram was performed after birth and revealed atrioseptal defect, mild tricuspid regurgitation, and small restrictive patent ductus arteriosus.
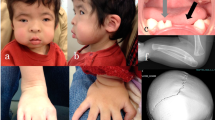
A comprehensive eye exam at the age of 4 days revealed bilateral microphthalmia (Axial length 12.5 mm bilaterally), clear but small corneas (OD 8.6 mm, OS 7 mm), right eye showed posterior embryotoxon and unresponsive small pupil, while the left eye showed correctopia of the iris with inferior ectropion uvea. Both lenses were opaque (Fig. 1A, B).
Anterior segment of right (A) and left (B) eyes demonstrating correctopia and cataract. C, D Ultrasound Biomicroscopy of both eyes showing shallow anterior chamber (two asterisks), angle closure 360 degrees (arrowhead) with synechia. Ciliary body is underdeveloped and anteriorly rotated (arrow). One asterisk demonstrates the corneas. E, F Fundus photos of both eyes revealing pigmented chorioretinal patches. Images A and B were obtained by surgical microscope during examinations under anesthesia. Images C and D were obtained by 10 MHz B-scan ultrasound Aviso S (Quantel Medical, Clermont-Ferrand,France). Images E and F were obtained by Phoenix Clinical ICON Paediatric Retinal Camera.
Further examinations after cataract extraction revealed peripheral anterior synechia between the iris and the cornea. Ultrasound Biomicroscopy (UBM) showed shallow anterior chamber angle closure 360 degrees with synechia. The ciliary body was underdeveloped and anteriorly rotated (Fig. 1C, D). A retinal exam showed pigmentary patches (Fig. 1E, F). The patient developed bilateral elevated intraocular pressure (up to 35 mmHg) and corneal edema at 12 weeks.
Physical examination upon admission revealed pigmentary changes on the abdomen, hemangioma on the right arm and bilateral mild hammertoe/camptodactyly of toes.
Further medical history included; bilateral chronic serous otitis media with tympanic membrane retraction grade I/Bilateral conductive hearing loss that was treated with bilateral myringotomy and ventilating tube insertion. The patient had asthma/Recurrent aspirational pneumonia due to difficulties swallowing liquids, treated with bronchodilators and specialized feeding.
Neurological examination revealed an asymmetric smile with flattening of the left nasolabial fold. Axial tonus was mildly low with mild head lag. Upon initial evaluation, global developmental delay was noted, particularly in motor milestones achievement. Neurological evaluation included electroencephalogram (EEG) that was normal and brain magnetic resonance imaging (MRI) at age one year and four months that showed mild bilateral ventriculomegaly. The patient had additional imaging studies, including renal ultrasound that detected mild unilateral hydronephrosis, and dental MRI at the age of one year and five months that detected a relatively small maxilla and underdeveloped maxillary sinuses/palatal asymmetry due to the position of yet erupted permanent teeth. Parents refused to perform dental x ray.
Molecular analysis
A genetic evaluation was performed using a commercial Next Generation Sequencing (NGS) targeted panel of 38 genes associated with congenital cataract, which included sequence and copy number variant analysis (AGK, BCOR, BFSP1, BFSP2, CHMP4B, CRYAA, CRYAB, CRYBA1, CRYBA4, CRYBB1, CRYBB2, CRYBB3, CRYGB, CRYGC, CRYGD, CRYGS, EPHA2, FAM126A, FOXC1, FYCO1, GALK1, GCNT2, GJA3, GJA8, HSF4, LIM2, MAF, MIP, NHS, OCRL, PAX6, PITX2, PITX3, SIL1, TDRD7, VIM, VSX2, Invitae - San Francisco, CA). Using DNA sample from peripheral blood, the analysis detected two heterozygous variants in BCOR gene (RefSeq NM_001123385) in a mosaic state: c.3463delG (p.Asp1155Thrfs*4) and c.3467delC (p.Pro1156Leufs*3), at a level of 28% (Coverage x452) and 24% (Coverage x421). The lab implemented both Short-Read (Illumina Inc. - San Diego, CA) and Long-Read (PacBio - Menlo Park, CA) NGS platforms, revealing these variants in trans, each on a different allele (Fig. 2A, B, respectively). Both variants were classified as “Pathogenic” accordingly to the ACMG/AMP 2015 guidelines.
Integrative Genome Viewer (IGV, Broad Institute, MA, USA) of short-reads (A) of the variant region at the BCOR gene demonstrated the existence of three different alleles; wild type (WT)/WT, WT/c.3463delG, and WT/c.3467delC. Confirmation using long-reads (B) sequencing, detected the existence of these three alleles.
Chromosomal Cytoscan 750 K Array (Affymetrix – Santa Clara, CA) has not revealed any copy number variants. The parents refused to proceed with additional tissue diagnosis.
Discussion
Our case is unique due to the severe ocular manifestations (phenotype) as it includes all parts of the eye globe. In contrast, most case series and reports in the literature report cataract with at most one or two more manifestations [3, 6,7,8,9,10,11,12]. A cataract is the most common ocular manifestation of OFCD, which may be unilateral or mild [7]. Microphthalmia is the second most common ocular finding in OFCD. It was described in 3 out of 10 patients in a series published by Redwood et al. [7] and in 27 out of 34 (79%) cases in a series by Hilton et al. [6]. Microcornea was described in 8 [3, 6, 11] out of 27 cases (29.6%) that were reviewed [3, 6,7,8,9,10,11,12]. The other ocular manifestations that were found in our case have been reported very rarely. Of 72 cases that were reviewed in the literature, embryotoxon [10], retinal pigmentary changes [9], and PFV [3] were reported only once, and correctopia was reported twice [6].
Our patient had developed bilateral glaucoma. Glaucoma in OFCD might be secondary to cataract extraction (aphakic glaucoma) and to microphthalmia but can also be attributed to the chronic angle closure, which was well demonstrated both by clinical exam and UBM.
Given the X-linked dominant trait of OFCD, variable expressivity is expected in females. Among the different mechanisms involved, this could be mainly attributed to the X-Inactivation pattern [4, 6, 10] as well as the existence of a somatic mosaic state in females [6, 10]. We show a female with a severe phenotype and mosaic heterozygous loss-of-function variants (“frameshift” mutations) on both alleles. This unique cellular condition leads to a situation in which the relative impact of the mutated alleles upon the phenotype is more significant, according with the level of mosaicism and independent of the X-Inactivation pattern. Therefore, the severity of the female phenotype due to biallelic somatic mosaicism is expected to resemble a germline mutation rather than presumed somatic mosaicism per se. As no males with OFCD/BCOR loss-of-function variants exist, it is expected that no females with biallelic pathogenic variants exist as well. However, this case demonstrates that biallelic mosaic variants are still compatible with life, thus implying the possibility of a dose-dependent pathogenic effect of the mutated allele cellular product.
This unique state explains the severity of the case presentation and widens the phenotype associated with BCOR-related conditions while providing an additional mechanism for the variable phenotype severity among females. The possibility of biallelic mosaicism in the BCOR gene should be thought of in cases of females presenting with a severe OFCD.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wilkie AO, Taylor D, Scambler PJ, Baraitser M. Congenital cataract, microphthalmia and septal heart defect in two generations: a new syndrome? Clin Dysmorphol. 1993;2:114–9.
Obwegeser HLGR. Oculo-facio-cardio-dental (OFCD) syndrome. Clin Dysmorphol. 1997;6:281–3.
Ng D, Thakker N, Corcoran CM, Donnai D, Parvin R, Schneider A, et al. Oculofaciocardiodental and Lenz microphthalmia syndromes result from distinct classes of mutations in BCOR. Nat Genet. 2004;36:411–6.
Hedera P, Gorski JL. Rapid publication oculo-facio-cardio-dental syndrome: skewed X chromosome inactivation in mother and daughter suggest X-linked dominant inheritance. Am J Med Genet. 2003;123:261–6.
Danda S, van Rahden VA, John D, Paul P, Raju R, Koshy SKK. Evidence of germline mosaicism for a novel BCOR mutation in two indian sisters with oculo-facio-cardio-dental syndrome. Mol Syndr. 2014;5:251–6.
Hilton E, Johnston J, Whalen S, Okamoto N, Hatsukawa Y, Nishio J, et al. BCOR analysis in patients with OFCD and Lenz microphthalmia syndromes, mental retardation with ocular anomalies, and cardiac laterality defects. Eur J Hum Genet. 2009;17:1325–35.
Redwood A, Douzgou S, Waller S, Ramsden S, Roberts A, Bonin H, et al. Congenital cataracts in females caused by BCOR mutations; report of six further families demonstrating clinical variability and diverse genetic mechanisms. Eur J Med Genet. 2020;63:103658.
Davoody A, Chen IP, Nanda R, Uribe F, Reichenberger EJ. Oculofaciocardiodental syndrome: a rare case and review of the literature. Cleft Palate Craniofacial J 2012;49:e55–e60.
Zhou Y, Wojcik A, Sanders VR, Rahmani B, Kurup SP. Ocular findings in a patient with oculofaciocardiodental (OFCD) syndrome and a novel BCOR pathogenic variant. Int Ophthalmol. 2018;38:2677–82.
Ragge N, Isidor B, Bitoun P, Odent S, Giurgea I, Cogné B, et al. Expanding the phenotype of the X-linked BCOR microphthalmia syndromes. Hum Genet. 2019;138:1051–69.
Gorlin RJ, Marashi AH, Obwegeser HL. Oculo-facio-cardio-dental (OFCD) syndrome. Am J Med Gene 1996;292:3–5.
Feberwee HE, Feenstra I, Oberoi S, Sama IE, Ockeloen CW, Clum F, et al. Letter to the Editor Novel BCOR mutations in patients with oculofaciocardiodental (OFCD) syndrome. Clin Genet. 2014;85:194–7.
Acknowledgements
We thank Dr. Ali Entezam and Dr. Swaroop Aradhya from Invitae Corp San Francisco, CA, USA for performing the molecular evaluation in this case.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors took active part in managing the patient and contributed to writing and reviewing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Tel Aviv Medical Center’s ethics committee exempted this research from review due to the descriptive nature of the case report. Informed consent was obtained from patient’s parents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mezad-Koursh, D., Rosenfeld, E., Bachar Zipori, A. et al. A rare genotype of biallelic mosaic variants in BCOR gene causing a bilateral ocular anterior segment dysgenesis and cataracts. Eur J Hum Genet 31, 125–127 (2023). https://doi.org/10.1038/s41431-022-01195-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-022-01195-7
This article is cited by
-
New year, new genes
European Journal of Human Genetics (2023)