Abstract
Devastating pandemics, such as that due to COVID-19, can provide strong testimony to our knowledge of the genetic and evolutionary determinants of infectious disease susceptibility and severity. One of the most remarkable aspects of such outbreaks is the stunning interindividual variability observed in the course of infection. In recent decades, enormous progress has been made in the field of the human genetics of infectious diseases, and an increasing number of human genetic factors have been reported to explain, to a great extent, the observed variability for a large number of infectious agents. However, our understanding of the cellular, molecular, and immunological mechanisms underlying such disparities between individuals and ethnic groups, remains very limited. Here, we discuss recent findings relating to human genetic predisposition to infectious disease, from an immunological or population genetic perspective, and show how these and other innovative approaches have been applied to deciphering the genetic basis of human susceptibility to COVID-19 and the severity of this disease. From an evolutionary perspective, we show how past demographic and selection events characterizing the history of our species, including admixture with archaic humans, such as Neanderthals, facilitated modern human adaptation to the threats imposed by ancient pathogens. In the context of emerging infectious diseases, these past episodes of genetic adaptation may contribute to some of the observed population differences in the outcome of SARS-CoV-2 infection and the severity of COVID-19 illness.
Similar content being viewed by others
Introduction
A striking observation in the current COVID-19 pandemic, as observed in several previous outbreaks of other diseases, is that infection with the causal agent (SARS-CoV-2 in this case) does not affect everyone equally. More than five million people have already died from COVID-19, as of February 2022, but death is disproportionately likely in certain individuals, including men and the elderly. After decades of intensive research in human genetics, it is now widely accepted that the large phenotypic variability observed in the course of infection is not random [1,2,3] and can stem, at least partly, from natural differences in the genetic make-up of humans [4, 5]. Our co-evolution with—and co-adaptation to—infectious agents over thousands of years has left molecular signatures in our genomes, which can still be partly disclosed through the use of population and evolutionary genetic approaches. Over the last decade, an increasing number of genes involved in immune functions and host-pathogen interactions have been identified as candidate targets of positive Darwinian selection due to the long-standing pressures imposed by pathogens [4, 6, 7]. Remarkably, such pressures may have important consequences for human health today, including greater resistance to emerging diseases, as testified by the iconic case of ancient selection at the CCR5 locus and present-day resistance to human immunodeficiency virus [8, 9]. However, selectively advantageous genetic variants are often unevenly distributed within and between populations, and may, thus, underlie interindividual heterogeneity in the outcome of infection. Furthermore, rare deleterious variants that disrupt or abolish the function of essential immune system genes, can exacerbate disparities in susceptibility to infectious disease.
In this review, we will focus on how human and population genetic approaches have facilitated the dissection of the molecular and cellular determinants of critical COVID-19. We will rapidly review how state-of-the-art knowledge on human predisposition to viral disease has led to the discovery of the strongest monogenic predispositions to COVID-19 pneumonia identified to date. We will also review the main loci identified in genome-wide association studies (GWAS) as underlying more complex, but also broader predispositions to COVID-19 at the population level. In this context, we will outline previous studies aiming to unravel the grounds for population differences in response to infection, and discuss them in the context of COVID-19. Finally, we will discuss how evolutionary genetics approaches have provided insight into the genetic determinants of COVID-19 susceptibility. Specifically, we will comment on the contribution of past admixture with Neanderthals to present-day susceptibility to viruses, and how particular DNA segments of Neanderthal origin present in the genomes of current non-African populations can alter modern human predisposition to severe COVID-19.
Monogenic predisposition to COVID-19
Over the last 2 years, with the support of unprecedented epidemiological observations of COVID-19 pathophysiology at a worldwide scale, researchers have sought to answer an intriguing question: why would a typically healthy child, teenager or young adult require admission to an intensive care unit following SARS-CoV-2 infection? Since the early twentieth century, epidemiological, genetic and molecular studies have garnered compelling evidence that human genetic factors play a fundamental role in determining the outcome of infectious disease [10,11,12]. Definitive proof that inborn errors of immunity (IEI)—rare genetic defects of the immune system—are related to an increase in susceptibility to an individual infectious agent was first provided in 1996, by studies demonstrating that IFN-γ receptor 1 deficiency underlies infections with non-virulent mycobacteria [13]. Predispositions to many infections have since been shown to be controlled by a narrow set of core genes, specific to each type of infection. For example, since 2015, it has been shown that life-threatening influenza pneumonia is linked to inborn errors of TLR3-, IRF7-, and IRF9-dependent type I interferon (IFN) immunity [14, 15]. These groundbreaking discoveries paved the way for the first breakthrough in dissecting the genetic basis of susceptibility to severe COVID-19.
A pioneering study led by the international COVID Human Genetic Effort consortium (www.covidhge.com), based on exome or genome sequencing data from 659 critically ill COVID-19 patients of various ancestries, revealed a significant enrichment in biochemically deleterious variants of the three core genes for influenza susceptibility (TLR3, IRF7 and IRF9) and ten other closely related viral genes (odds ratio (OR) = 8.28; p = 0.01) in these patients, relative to 534 subjects with asymptomatic or benign infections [16]. The genetic homogeneity underlying susceptibility to influenza and COVID-19 was not particularly surprising, given that both these conditions are respiratory infections caused by RNA viruses and transmitted by droplets and small airborne particles.
Independently of influenza, the essential role of type I IFNs in COVID-19 pathogenesis was supported by the discovery that critical COVID-19 pneumonia in 16 unrelated men carrying deleterious X-linked TLR7 variants was caused by the low type I IFN production of their plasmacytoid dendritic cells in response to SARS-CoV-2 [17]. Yet, other studies have brought supporting evidence for the implication of type I IFN-independent genes as monogenic etiologies of COVID-19. For example, genetic variants in the CFTR gene, including those causing cystic fibrosis, have been found to be overrepresented among critically ill COVID-19 patients [18,19,20], consistent with previous associations of the gene with susceptibility to respiratory tract infections [21]. Likewise, a genetic polymorphism in the androgen receptor (AR) gene that correlates with low serum testosterone level has been associated with severe COVID-19 in men [22]. However, immunological and clinical data supporting the implication of these type I IFN-independent genes in COVID-19 pathogenesis remain circumstantial.
Further evidence for the pervasive role of type I IFNs in modulating COVID-19 severity was provided by multiple independent observations showing that many individuals with critical disease carry autoantibodies neutralizing type I IFNs [23,24,25,26], a phenocopy of the inborn errors. This observation was in line with previous studies describing autoimmune phenocopies of IEI for type II IFN, IL-6, IL-17A/F and granulocyte-macrophage colony-stimulating factor, underlying mycobacterial disease, staphylococcal disease, mucocutaneous candidiasis and nocardiosis, respectively. Importantly, in all cases in which data were available, the autoantibodies were found to have been present before SARS-CoV-2 infection, supporting the notion that they are the cause rather than a consequence of the disease [16]. The contribution of these studies to improving our understanding of the genetic architecture of COVID-19 severity has been such that it is now estimated that ~20% of patients with critical COVID-19 over 80 years of age, and ~20% of patients of all ages who died from the disease, carried autoantibodies neutralizing type I IFNs. Interestingly, a subsequent study showed that the prevalence of type I IFN autoantibodies in the general population was higher in men than in women [27]. This, together with the findings related to the TLR7 [17] and AR [22] genes, provides cues as to the genetic and biological mechanisms underlying the observed sex bias among critical COVID-19 cases [28]. Furthermore, it was noted that the prevalence of type I IFN autoantibodies increases significantly with age [27], consistent with the early-established higher risk of death from COVID-19 in the elderly population [28]. Studies based on clinical genetic approaches have, thus, shown that IEI and their autoimmune phenocopies contribute to the pathogenesis of about 15–20% of patients with critical COVID-19 pneumonia, representing a major burden in individuals over 80 years old.
Complex predisposition to COVID-19
By contrast to the strong allelic effects of IEI as monogenic determinants of COVID-19 illness (ORs typically >5), common variants (frequency >5%) are expected to underlie more subtle, complex patterns of susceptibility (ORs <1.5), usually orchestrated by several genes. Other international efforts were established early in the pandemic to determine the contribution of common variants to either susceptibility to infection with SARS-CoV-2 or to COVID-19 severity. Specifically, the COVID-19 Host Genetics Initiative (COVID-19 HGI), GenOMICC (Genetics Of Mortality In Critical Care), the 23andMe COVID-19 Team and the Severe Covid-19 GWAS Group conducted GWAS with an unprecedented number of cases and controls in the field of infectious disease genomics research [29,30,31,32]. The largest study to date, a meta-analysis including 125,584 cases and over 2.5 million controls across 60 studies from 25 countries conducted by COVID-19 HGI, has identified 23 loci significantly associated with disease severity or susceptibility to infection [33]. These loci include a region on chromosome 3 (3p21.31) and the ABO locus, these genomic regions being the most frequently replicated to date (six and five times, respectively). The most promising evidence concerning the greater susceptibility to SARS-CoV-2 infection associated with the 3p21.31 locus points to involvement of the SLC6A20 gene, encoding a sodium transporter interacting with ACE2, the well-known receptor for SARS-CoV-2 on the cell surface [34]. Consistent with this, the most recent analyses performed by the COVID-19 HGI have identified a polymorphism (rs190509934) close to the ACE2 gene that is known to lower ACE2 expression [35] and is associated with a lower risk of infection. Interestingly, an earlier study showed a significant association between ACE2 allelic variation and COVID-19 severity [36]. The ABO locus has also been found to be more strongly associated with susceptibility to infection rather than COVID-19 severity [29], with blood groups O and A protecting against and increasing the risk of infection, respectively.
The remaining significant hits in GWAS have been less frequently replicated, but the associations of IFNAR2 and TYK2 with COVID-19 severity [29, 30] merit discussion, given the key immunoregulatory functions of the proteins they encode. A potential role of IFNAR2, encoding the second chain of the type I IFN receptor, in modulating disease severity is consistent with the known role of type I IFNs in protection against COVID-19 severity. A similar, but more controversial finding is the association of TYK2 variants with critical COVID-19, with the rs34536443 variant reportedly causal for the underlying phenotype [29]. The suggested causal role of rs34536443 is supported by its association with protection against multiple autoimmune disorders [37] and the established link between complete TYK2 deficiency and susceptibility to severe recurrent infections [38]. However, the rs34536443-CC genotype has been shown to selectively impair cellular responses to IL-23 but not those to IFN-α or IL-10, consistent with rs34536443 being a common monogenic etiology of tuberculosis but not of viral infectious diseases [39, 40]. Finally, albeit the variant has not been found by GWAS, a common TLR3 missense variant, impairing perhaps type I IFN immunity, has been reported as a marker of COVID-19 severity [41].
GWAS have identified other genetic variants associated with either critical illness or susceptibility to infection with SARS-CoV-2, including variants in the OAS-RNase L and DPP4-DPP9 clusters, which we will discuss later, MUC5B and FOXP4, previously associated with lung-related phenotypes, and three human leukocyte antigens (HLA) binding to epitope peptides prompting pathogen recognition by the immune system [29, 30]. HLA-G rs9380142 and rs143334143, in the vicinity of HLA-C, were found to be associated with COVID-19 severity in both the HGI and GenOMICC studies, whereas HLA-DPB1 rs2071351 reached genome-wide significance in the susceptibility analysis of the HGI. Many of these GWAS hits have already been identified in previous studies of lung-related phenotypes, autoimmune or inflammatory diseases [37, 42,43,44], but further replications and analyses of the underlying cellular mechanisms are required to improve our understanding of the clinical value of these findings in the context of COVID-19.
Genetic ancestry and differences in COVID-19 susceptibility
The human genetic factors reported to be associated with COVID-19 in GWAS, despite the uneven representation of different ancestries in these studies, are not equally distributed between populations across the globe. We can therefore wonder whether, as with age, sex or socioeconomic status [45, 46], genetic ancestry, which reflects past differences in population demographic history and adaptation, can also help to explain COVID-19-related health disparities between populations of individuals from different ethnic backgrounds. This is probably not the case for individuals presenting with IEI, who are, by definition, immunocompromised, a notion extending beyond the differences imposed by geographic barriers. For COVID-19, this is consistent with the high risk (OR > 8) associated with the carriage of monogenic lesions in type I IFN-related genes [16] and the observation that the two strongest genetic associations reported by the trans-ancestry analysis of the 23andMe COVID-19 Team at the ABO and 3p21.31 loci did not explain differences in risk between populations [32]. However, the same study also showed that non-European ancestry was a significant risk factor for hospitalization, after accounting for sociodemographic factors and pre-existing health conditions, supporting the notion that complex genetic architectures, as opposed to strong allelic effects, may account for population-level differences in the outcome of SARS-CoV-2 infection. In support of this hypothesis, efforts to quantify differences between ancestries in rates of SARS-CoV-2 infection and COVID-19 clinical manifestations in England showed that ethnic minorities had higher risks of testing positive for SARS-CoV-2 and of adverse COVID-19 outcomes, after accounting for differences in sociodemographic, clinical, and household characteristics [47].
More generally, these observations are supported by previous studies exploring the extent and nature of population differences in immune responses [48, 49]. For example, differential transcriptional responses to viral and bacterial stimuli have been described between individuals of African and European descent. More recently, a single-cell RNA-sequencing study of influenza-infected immune cells from individuals of European and African ancestry reported ancestry-dependent gene signatures under the control of human genetic factors (here, cis-expression quantitative trait loci, eQTLs) differing between ancestries [50]. Interestingly, the overlap in the immune system genes underlying susceptibility to both influenza and COVID-19 suggests that a similar situation may apply to COVID-19, although single-cell studies investigating population differences in immune responses to SARS-CoV-2 infection are still lacking.
The underlying causes of such differences in immunity post-infection between ethnic groups can also be investigated by adopting a different strategy, based on population and evolutionary genetics approaches. In this context, one recent study sought to quantify the impact of past coronavirus-like epidemics across the globe, by screening for signatures of selection at loci involving 420 coronavirus-interacting human proteins (CoV-VIPs) in different populations [51]. Surprisingly, none of the 26 populations examined worldwide presented a signature of adaptation at the CoV-VIP loci, with the exception of populations of east Asian ancestry, consistent with the geographic origin of several modern coronavirus epidemics. In short, the authors found a cluster of 42 CoV-VIP loci displaying consistent patterns of adaptive evolution, dating back to 20,000 years ago, in East Asian populations, reflecting long-term selection pressures exerted by coronavirus-like viruses on the ancestors of modern-day East Asians. Possible geographically restricted positive selection at immune loci has also been detected in a Japanese population, in which the DPB1*04:01 HLA allele was found to have undergone a strong recent increase in frequency [52]. In line with a putative protective effect of this variant against hepatitis B virus infection [53], the authors speculated that this increase in frequency in the Japanese population resulted from past pathogen-driven selection. Collectively, these studies support a role for local human adaptation in response to past and present infectious agents in increasing immune response disparities between populations around the world.
Ancient admixture with Neanderthals and RNA viruses
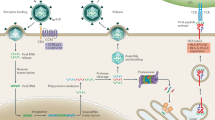
The observed differences in the genetic make-up of populations from different ancestries result not only from their past demographic or adaptive history, but also from differences in their past history of admixture (or hybridization) with other types of humans that are now extinct (Fig. 1). Anatomically modern humans interbred with these archaic hominins, such as Neanderthals and Denisovans, on multiple occasions and in several locations [54]. As a result, all non-African groups share ∼2% Neanderthal ancestry in their genomes, whereas some south-east Asian and Oceanian populations have accumulated up to 5–6% of combined (Neanderthal and Denisovan) archaic ancestry [55]. There is evidence to suggest that purifying selection has been the dominant selective force acting against the introgression of archaic DNA material, leading to a steady decrease in haplotypes of an archaic nature in the genomes of modern humans over time [56].
Graphical representation of the contribution of admixture with archaic humans and exposure to ancient viruses to differences in the response to infection between present-day populations. On the left of the figure, genetic material from archaic humans, such as Neanderthals or Denisovans, is shown to be inherited by non-Africans, a process that began ~50,000 years ago. When beneficial, this event is known as “adaptive introgression”, and is thought to have facilitated the acquisition of advantageous variants by modern humans, accelerating their adaptation to Eurasian pathogens (“pathogen group E”), which here are hypothesized to be different from African pathogens (“pathogen group A”). Continuous high-level exposure of the ancestors of modern East Asians to coronaviruses over the last ~20,000 years has left signatures of selection at CoV-VIP loci in the genomes of modern East Asians [51], a pattern that is not observed in other human populations. Together with other genetic or environmental factors, these historical events underlie some of the disparities observed today in predisposition to COVID-19 between human populations. Created with BioRender.com.
However, in some cases, hybridization events appear to have facilitated the acquisition of advantageous traits, a phenomenon known as “adaptive introgression” [57]. Neanderthals and Denisovans inhabited Eurasia for at least 300,000 years before modern humans arrived and are thought to have become genetically adapted to their local climates, nutritional resources and pathogens over this period. Unsurprisingly, there is increasing evidence to suggest that archaic introgression has facilitated the acquisition, by modern humans, of beneficial variants of immunity-related genes, attesting to the long-term adaptation of the archaic species to pathogens outside of Africa. Interestingly, an early, influential work showed an enrichment in Neanderthal ancestry among innate immunity genes in Europeans [58]. Similarly, high levels of Neanderthal or Denisovan ancestry have been detected in the genomes of modern humans at loci including the antiviral OAS genes, the TLR1-6-10 gene cluster or the inflammation-related TNFAIP3 gene in several non-African populations around the world [59,60,61].
Remarkably, two studies found an enrichment in Neanderthal ancestry for genetic variants associated with gene expression variation, eQTLs, in monocytes and macrophages in Europeans. This enrichment was particularly evident for genetic variants associated with antiviral responses [48, 49], suggesting that Neanderthal introgression, in particular, facilitated the genetic adaptation of early Eurasians to viral challenges. Consistent with this theory, human genes encoding proteins that interact with viruses were also found to display significantly high levels of Neanderthal ancestry, particularly those encoding proteins interacting with RNA viruses, such as influenza, hepatitis C virus or coronaviruses [62]. Another study explored the impact of Neanderthal ancestry on the human regulatory genetic landscape, including promoters, enhancers and miRNA-mediated regulation [63]. A massive colocalization of Neanderthal variants with active enhancers in adipose-related tissues and various types of primary T cells was observed. Collectively, these studies shed light on the extent to which archaic introgression has contributed to modern human adaptation to new environments, by modulating human immunity to newly encountered pathogens, including RNA viruses in particular.
Neanderthal heritage and the current COVID-19 pandemic
Recent studies driven by the COVID-19 pandemic have provided additional support for the links between Neanderthal introgression and human immunity to viruses. Two GWAS hits from the aforementioned human genetic determinants of COVID-19 susceptibility and severity overlap with genomic regions inherited from Neanderthals. One of these regions includes genetic variants at the chr12q24.13 locus, which are mostly absent from Africans but were present in Neanderthals. These variants define a ~75 kb haplotype in individuals of European ancestry that has been found to be associated with a 22% lower risk of hospitalization for COVID-19 [29, 30, 64]. The locus concerned covers the OAS-RNase L cluster, which encodes enzymes essential for antiviral immunity [65, 66]. However, the cellular mechanism underlying this improvement in COVID-19 outcomes is not yet fully understood. In this context, one recent study sought to delineate the causal variant associated with COVID-19 protection from more than a hundred linked variants within the same associated haplotype, by focusing on the rs10774671 variant, a candidate OAS1 splice acceptor variant [67]. The authors tested associations in different ancestry groups with markedly different levels of linkage disequilibrium (LD). By focusing on individuals of African ancestry, in whom LD levels are lowest and rs10774671 segregates independently of the other variants, the authors were able to identify a causal connection between the rs10774671-G allele and COVID-19 illness. These findings highlight the role of the OAS1 isoform p46, encoded by the splice site variant at this locus, in effective protection against hospitalization for COVID-19, at least in individuals of European or African descent (OR = 0.92–0.94, p = 5.8 × 10−10 − 0.03).
In stark contrast, the other COVID-19-related genomic region of Neanderthal origin, a locus on chromosome 3, 3p21.31, has been associated with greater susceptibility to the development of severe forms of COVID-19. It spans a 50-kb haplotype containing six genes (SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6 and XCR1). The contribution of one of these genes, SLC6A20, to susceptibility to infection has already been discussed. GWAS data suggest that the risk of hospitalization for COVID-19 is 60% higher in carriers of the Neanderthal haplotype, which is at least three times more frequent in individuals of South Asian descent (>50%) than in individuals with European ancestry (16%) [68]. This, together with unaccounted sociodemographic factors, may partly explain the higher risk of infection or hospitalization for COVID-19 in minorities of South Asian ancestry living in the UK [47]. Two independent studies aiming to determine the cellular basis of the increase in the risk of severe disease associated with this locus have suggested that this outcome may result from a decrease in CXCR6 levels [69, 70].
Finally, a genetic variant in the promoter region of the DPP4 gene, encoding a receptor for another coronavirus, MERS-CoV, but not for SARS-CoV-2, was also inherited from Neanderthals and has been shown to double the risk of developing critical COVID-19. The Neanderthal variant did not reach genome-wide significance in GWAS studies, but variants of DPP9, a homolog of DPP4, are, on the contrary, significantly associated with severe COVID-19 in GWAS, supporting a potential role for DPP4 in COVID-19 pathogenesis [30]. Together, these observations lend further support to the notion that Neanderthal introgression has had profound consequences for the adaptation of our species to viral challenges, and that such past adaptation events can affect the present-day health status of individuals infected with SARS-CoV-2.
Conclusions
The COVID-19 pandemic has had devasting humanitarian consequences. Vaccines were rapidly developed and have prevented the most harmful outcomes of infection with the virus. Nevertheless, the “miracle” brought about by vaccines simply reinforces the importance of prior decades of basic scientific research, without which no amount of investment could have provided us with better solutions so rapidly. In this respect, the field of human genetics of infectious diseases, driven by clinical, population and evolutionary genetic studies, has made spectacular breakthroughs over the last decade. Prompted by duty, and perhaps a fear of COVID-19, the scientific community has rapidly organized unprecedented international efforts, and provided scientific results more successfully and rapidly than ever before. For example, the discovery that the pathogenesis of the disease in 20% of patients with critical COVID-19 pneumonia can be explained by either IEIs of type I IFN immunity or pre-existing autoantibodies neutralizing type I IFNs is an outstanding finding for common infections, for which monogenic lesions have never been shown to underlie more than 1% of cases for other conditions [39]. The contributions of GWAS have also been substantial, particularly given the unprecedent number of cases (125,000) and controls (2.5 million) recruited for such studies, the second largest infectious GWAS in terms of case numbers being that performed by the 23andMe consortium on 107,769 cases of chickenpox and 15,982 controls [71].
Importantly, trans-ancestry analyses in GWAS have revealed population disparities in terms of susceptibility to infection or disease severity. The strongest genetic determinants of COVID-19 reported to date have similar effects on individuals across the globe, but non-European ancestries have been shown to confer a higher risk of developing severe forms of COVID-19. These studies have once again highlighted the importance of including diverse and underrepresented human populations in genomic studies, to delineate variants differing between ancestries that may, under complex genetic architectures, underlie population differences in disease outcome [72]. Such ancestry-inclusive efforts are of major importance in the context of drug development, as drug efficiency may depend strongly on the genetic make-up of the population. An eQTL study in the context of influenza infection in populations of African and European ancestry has already supported the notion that, in some conditions, variants differentially represented across ancestries can result in different genetic signatures, possibly attesting to an ancestry-specific activation of biological pathways [50]. Finally, the sequencing of archaic hominin genomes has made it possible to unravel some of the essential features of immunity responsible for facilitating the adaptation of early non-Africans to newly encountered pathogenic environments. Studies of the genetic legacy of archaic hominins in the genomes of modern humans extend well beyond questions relating to molecular anthropology, as attested by the surprising finding that the genetic legacy of ancient admixture with Neanderthals around 50,000 years ago still affects the health of humans today, even in the specific context of the COVID-19 pandemic.
References
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62.
Casanova JL, Abel L. Inborn errors of immunity to infection: the rule rather than the exception. J Exp Med. 2005;202:197–201.
Quintana-Murci L. Human immunology through the lens of evolutionary genetics. Cell. 2019;177:184–99.
Casanova JL, Abel L, Quintana-Murci L. Immunology taught by human genetics. Cold Spring Harb Symp Quant Biol. 2013;78:157–72.
Barreiro LB, Quintana-Murci L. From evolutionary genetics to human immunology: how selection shapes host defence genes. Nat Rev Genet. 2010;11:17–30.
Fumagalli M, Sironi M, Pozzoli U, Ferrer-Admetlla A, Pattini L, Nielsen R. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet. 2011;7:e1002355.
Lalani AS, Masters J, Zeng W, Barrett J, Pannu R, Everett H, et al. Use of chemokine receptors by poxviruses. Science. 1999;286:1968–71.
Bamshad MJ, Mummidi S, Gonzalez E, Ahuja SS, Dunn DM, Watkins WS, et al. A strong signature of balancing selection in the 5’ cis-regulatory region of CCR5. Proc Natl Acad Sci USA. 2002;99:10539–44.
Dubos RJ. Second thoughts on the germ theory. Sci Am. 1955;192:31–5.
Allison AC. Protection afforded by sickle-cell trait against subtertian malareal infection. Br Med J. 1954;1:290–4.
Casanova JL, Abel L. Lethal infectious diseases as inborn errors of immunity: toward a synthesis of the germ and genetic theories. Annu Rev Pathol. 2021;16:23–50.
Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guérin infection. N Engl J Med. 1996;335:1956–61.
Ciancanelli MJ, Huang SX, Luthra P, Garner H, Itan Y, Volpi S, et al. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science. 2015;348:448–53.
Casanova JL, Abel L. The human genetic determinism of life-threatening infectious diseases: genetic heterogeneity and physiological homogeneity? Hum Genet. 2020;139:681–94.
Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570.
Asano T, Boisson B, Onodi F, Matuozzo D, Moncada-Velez M, Maglorius Renkilaraj MRL, et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci Immunol. 2021;6:eabl4348.
Baldassarri M, Fava F, Fallerini C, Daga S, Benetti E, Zguro K, et al. Severe COVID-19 in hospitalized carriers of single CFTR pathogenic variants. J Pers Med. 2021;11:558.
Corvol H, de Miranda S, Lemonnier L, Kemgang A, Reynaud Gaubert M, Chiron R, et al. First wave of COVID-19 in French patients with cystic fibrosis. J Clin Med. 2020;9:3624.
Fallerini C, Picchiotti N, Baldassarri M, Zguro K, Daga S, Fava F, et al. Common, low-frequency, rare, and ultra-rare coding variants contribute to COVID-19 severity. Hum Genet. 2022;141:147–73.
Miller AC, Comellas AP, Hornick DB, Stoltz DA, Cavanaugh JE, Gerke AK, et al. Cystic fibrosis carriers are at increased risk for a wide range of cystic fibrosis-related conditions. Proc Natl Acad Sci USA. 2020;117:1621–7.
Baldassarri M, Picchiotti N, Fava F, Fallerini C, Benetti E, Daga S, et al. Shorter androgen receptor polyQ alleles protect against life-threatening COVID-19 disease in European males. EBioMedicine. 2021;65:103246.
Chauvineau-Grenier A, Bastard P, Servajean A, Gervais A, Rosain J, Jouanguy E, et al. Autoantibodies neutralizing type I interferons in 20% of COVID-19 deaths in a French hospital. J Clin Immunol 2022;42:459–70.
Troya J, Bastard P, Planas-Serra L, Ryan P, Ruiz M, de Carranza M, et al. Neutralizing autoantibodies to type I IFNs in >10% of patients with severe COVID-19 pneumonia hospitalized in Madrid, Spain. J Clin Immunol. 2021;41:914–22.
Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585.
Koning R, Bastard P, Casanova JL, Brouwer MC, van de Beek D. Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 2021;47:704–6.
Bastard P, Gervais A, Le Voyer T, Rosain J, Philippot Q, Manry J, et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci Immunol. 2021;6:eabl4340.
Ahrenfeldt LJ, Otavova M, Christensen K, Lindahl-Jacobsen R. Sex and age differences in COVID-19 mortality in Europe. Wien Klin Wochenschr. 2021;133:393–8.
COVID-19 Host Genetics Initiative.Mapping the human genetic architecture of COVID-19. Nature. 2021;600:472–7.
Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D, et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591:92–8.
Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020;383:1522–34.
Shelton JF, Shastri AJ, Ye C, Weldon CH, Filshtein-Sonmez T, Coker D, et al. Trans-ancestry analysis reveals genetic and nongenetic associations with COVID-19 susceptibility and severity. Nat Genet. 2021;53:801–8.
COVID-19 Host Genetics Initiative, Ganna A. Mapping the human genetic architecture of COVID-19: an update. medRxiv. 2021. https://doi.org/10.1101/2021.11.08.21265944.
Vuille-dit-Bille RN, Camargo SM, Emmenegger L, Sasse T, Kummer E, Jando J, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47:693–705.
Horowitz JE, Kosmicki JA, Damask A, Sharma D, Roberts GHL, Justice AE, et al. Genome-wide analysis in 756,646 individuals provides first genetic evidence that ACE2 expression influences COVID-19 risk and yields genetic risk scores predictive of severe disease. medRxiv. 2021.
Benetti E, Tita R, Spiga O, Ciolfi A, Birolo G, Bruselles A, et al. ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. Eur J Hum Genet. 2020;28:1602–14.
Dendrou CA, Cortes A, Shipman L, Evans HG, Attfield KE, Jostins L, et al. Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci Transl Med. 2016;8:363ra149.
Kreins AY, Ciancanelli MJ, Okada S, Kong XF, Ramírez-Alejo N, Kilic SS, et al. Human TYK2 deficiency: mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med. 2015;212:1641–62.
Kerner G, Ramirez-Alejo N, Seeleuthner Y, Yang R, Ogishi M, Cobat A, et al. Homozygosity for TYK2 P1104A underlies tuberculosis in about 1% of patients in a cohort of European ancestry. Proc Natl Acad Sci USA. 2019;116:10430–4.
Boisson-Dupuis S, Ramirez-Alejo N, Li Z, Patin E, Rao G, Kerner G, et al. Tuberculosis and impaired IL-23-dependent IFN-γ immunity in humans homozygous for a common TYK2 missense variant. Sci Immunol. 2018;3:eaau8714.
Croci S, Venneri MA, Mantovani S, Fallerini C, Benetti E, Picchiotti N, et al. The polymorphism L412F in TLR3 inhibits autophagy and is a marker of severe COVID-19 in males. Autophagy. 2021:1–11. Online ahead of print.
Dai J, Lv J, Zhu M, Wang Y, Qin N, Ma H, et al. Identification of risk loci and a polygenic risk score for lung cancer: a large-scale prospective cohort study in Chinese populations. Lancet Respir Med. 2019;7:881–91.
Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45:613–20.
Manichaikul A, Wang XQ, Sun L, Dupuis J, Borczuk AC, Nguyen JN, et al. Genome-wide association study of subclinical interstitial lung disease in MESA. Respir Res. 2017;18:97.
de Glanville WA, Thomas LF, Cook EAJ, Bronsvoort BMC, Wamae NC, Kariuki S, et al. Household socio-economic position and individual infectious disease risk in rural Kenya. Sci Rep. 2019;9:2972.
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–6.
Mathur R, Rentsch CT, Morton CE, Hulme WJ, Schultze A, MacKenna B, et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. 2021;397:1711–24.
Nédélec Y, Sanz J, Baharian G, Szpiech ZA, Pacis A, Dumaine A, et al. Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell. 2016;167:657–69.e21.
Quach H, Rotival M, Pothlichet J, Loh YE, Dannemann M, Zidane N, et al. Genetic adaptation and Neandertal admixture shaped the immune system of human populations. Cell. 2016;167:643–56.e17.
Randolph HE, Fiege JK, Thielen BK, Mickelson CK, Shiratori M, Barroso-Batista J, et al. Genetic ancestry effects on the response to viral infection are pervasive but cell type specific. Science. 2021;374:1127–33.
Souilmi Y, Lauterbur ME, Tobler R, Huber CD, Johar AS, Moradi SV, et al. An ancient viral epidemic involving host coronavirus interacting genes more than 20,000 years ago in East Asia. Curr Biol. 2021;31:3504–14.e9.
Kawashima M, Ohashi J, Nishida N, Tokunaga K. Evolutionary analysis of classical HLA class I and II genes suggests that recent positive selection acted on DPB1*04:01 in Japanese population. PLoS ONE. 2012;7:e46806.
Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, Hosono N, et al. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet. 2009;41:591–5.
Dannemann M, Racimo F. Something old, something borrowed: admixture and adaptation in human evolution. Curr Opin Genet Dev. 2018;53:1–8.
Choin J, Mendoza-Revilla J, Arauna LR, Cuadros-Espinoza S, Cassar O, Larena M, et al. Genomic insights into population history and biological adaptation in Oceania. Nature. 2021;592:583–9.
Harris K, Nielsen R. The genetic cost of Neanderthal introgression. Genetics. 2016;203:881–91.
Taylor SA, Larson EL. Insights from genomes into the evolutionary importance and prevalence of hybridization in nature. Nat Ecol Evol. 2019;3:170–7.
Deschamps M, Laval G, Fagny M, Itan Y, Abel L, Casanova JL, et al. Genomic signatures of selective pressures and introgression from Archaic Hominins at human innate immunity genes. Am J Hum Genet. 2016;98:5–21.
Dannemann M, Andrés AM, Kelso J. Introgression of Neandertal- and Denisovan-like haplotypes contributes to adaptive variation in human toll-like receptors. Am J Hum Genet. 2016;98:22–33.
Sams AJ, Dumaine A, Nédélec Y, Yotova V, Alfieri C, Tanner JE, et al. Adaptively introgressed Neandertal haplotype at the OAS locus functionally impacts innate immune responses in humans. Genome Biol. 2016;17:246.
Zammit NW, Siggs OM, Gray PE, Horikawa K, Langley DB, Walters SN, et al. Denisovan, modern human and mouse TNFAIP3 alleles tune A20 phosphorylation and immunity. Nat Immunol. 2019;20:1299–310.
Enard D, Petrov DA. Evidence that RNA viruses drove adaptive introgression between Neanderthals and modern humans. Cell. 2018;175:360–71.e13.
Silvert M, Quintana-Murci L, Rotival M. Impact and evolutionary determinants of Neanderthal introgression on transcriptional and post-transcriptional regulation. Am J Hum Genet. 2019;104:1241–50.
Zeberg H, Pääbo S. A genomic region associated with protection against severe COVID-19 is inherited from Neandertals. Proc Natl Acad Sci USA. 2021;118:e2026309118.
Choi UY, Kang JS, Hwang YS, Kim YJ. Oligoadenylate synthase-like (OASL) proteins: dual functions and associations with diseases. Exp Mol Med. 2015;47:e144.
Gresser I, Vignaux F, Belardelli F, Tovey MG, Maunoury MT. Injection of mice with antibody to mouse interferon alpha/beta decreases the level of 2’-5’ oligoadenylate synthetase in peritoneal macrophages. J Virol. 1985;53:221–7.
Huffman JE, Butler-Laporte G, Khan A, Pairo-Castineira E, Drivas TG, Peloso GM, et al. Multi-ancestry fine mapping implicates OAS1 splicing in risk of severe COVID-19. Nat Genet. 2022;54:125–7.
Zeberg H, Pääbo S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature. 2020;587:610–2.
Dai Y, Wang J, Jeong HH, Chen W, Jia P, Zhao Z. Association of CXCR6 with COVID-19 severity: delineating the host genetic factors in transcriptomic regulation. Hum Genet. 2021;140:1313–28.
Katz DH, Tahir UA, Ngo D, Benson MD, Bick AG, Pampana A, et al. Proteomic profiling in biracial cohorts implicates DC-SIGN as a mediator of genetic risk in COVID-19. medRxiv. 2020.
Tian C, Hromatka BS, Kiefer AK, Eriksson N, Noble SM, Tung JY, et al. Genome-wide association and HLA region fine-mapping studies identify susceptibility loci for multiple common infections. Nat Commun. 2017;8:599.
Sirugo G, Williams SM, Tishkoff SA. The missing diversity in human genetic studies. Cell. 2019;177:1080.
Acknowledgements
We thank Yann Aquino and Sebastian Cuadros Espinoza for helpful discussions.
Funding
The Laboratory of Human Evolutionary Genetics is supported by the Institut Pasteur, the Collège de France, the Centre Nationale de la Recherche Scientifique (CNRS), the Agence Nationale de la Recherche (ANR) grants CNSVIRGEN (ANR-19-CE15-0009-02), COVID-19-POPCELL (ANR-21-CO14-0003-01) and COVIFERON (ANR-21-RHUS-0008), the French Government’s Investissement d’Avenir program, Laboratoires d’Excellence “Integrative Biology of Emerging Infectious Diseases” (ANR-10- LABX-62-IBEID) and “Milieu Intérieur” (ANR-10-LABX-69-01), the Fondation pour la Recherche Médicale (Equipe FRM DEQ20180339214), the Fondation Allianz-Institut de France, the Fondation de France (no. 00106080), and the EU Horizon Europe Framework Programme (HORIZON-HLTH-DISEASE-2021-04-07-UNDINE). GK is supported by a Pasteur-Roux-Cantarini fellowship.
Author information
Authors and Affiliations
Contributions
All authors conceptualized and wrote the manuscript, critically revised it, and approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kerner, G., Quintana-Murci, L. The genetic and evolutionary determinants of COVID-19 susceptibility. Eur J Hum Genet 30, 915–921 (2022). https://doi.org/10.1038/s41431-022-01141-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-022-01141-7
This article is cited by
-
Next-generation sequencing of host genetics risk factors associated with COVID-19 severity and long-COVID in Colombian population
Scientific Reports (2024)
-
2022: the year that was in the European Journal of Human Genetics
European Journal of Human Genetics (2023)
-
COVID-19: a challenge and an opportunity
European Journal of Human Genetics (2022)