Abstract
Krabbe disease (KD) is a rare lysosomal storage disorder caused by biallelic pathogenic variants in GALC. Most patients manifest the severe classic early-infantile form, while a small percentage of cases have later-onset types. We present two siblings with atypical clinical and neuroimaging phenotypes, compared to the classification of KD, who were found to carry biallelic loss-of-function GALC variants, including a recurrent 30 kb deletion and a previously unreported deep intronic variant that was identified by mRNA sequencing. This family represents a unique description in the KD literature and contributes to expanding the clinical and molecular spectra of this rare disorder.
Similar content being viewed by others
Introduction
Globoid cell leukodystrophy or Krabbe disease (KD) is a rare lysosomal storage disorder caused by mutations in GALC. It is characterized by deficiency of the hydrolytic lysosomal enzyme galactocerebrosidase, which catabolizes the major myelin lipid galactosylceramide during normal myelin turnover. The galactocerebrosidase is also responsible for degradation of galactosylsphingosine (psychosine), an intermediate in the biosynthesis of cerebrosides, whose accumulation causes abnormal turnover of myelin and death of both oligodendrocytes and Schwann cells [1, 2]. KD represents a spectrum ranging from the severe (early) infantile- (<12 months) to late-onset forms [1, 3]. Here we present two siblings sharing compound heterozygosity for two GALC variants, who showed different onset and course of disease as well as atypical clinical and neuroimaging phenotypes, which do not fit with current classification of known KD forms.
Subjects and methods
We retrospectively describe main data of two affected sibs with biallelic GALC variants.
The study was conducted according to the guidelines of the Declaration of Helsinki. Parents of the involved patients provided written informed consent for molecular analyses and study participation. Please refer to Supplementary file for methodology of molecular analyses and galactocerebrosidase activity assay.
Results
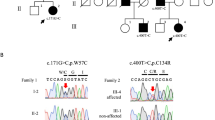
The older sister is an 11-year-old girl born after uneventful pregnancy from cesarean section due to fetal dystocia. She had normal neonatal period and gained normal motor and cognitive milestones in the first year. She moved first steps on tiptoes at age 14 months. First neurological examination at age 18 months detected a spastic paraparesis and poor growth (weight <3%, length 10–25%). At this time, brain and spine magnetic resonance imaging (MRI) detected mild supratentorial white matter (WM) hyperintensities (Fig. 1A–C), nerve conduction study (NCS) revealed demyelinating sensory-motor neuropathy and full metabolic screening was unrevealing. Over the years, several laboratory tests have been repeated including hexosylsphingosines (HexSph) determination, which was in the normal range. Serial clinical assessment showed slowly progressive worsening of pyramidal syndrome and also worsening of WM lesions was documented by neuroimaging follow-up at age 4 (not shown), 9 and 10 (Fig. 1D–I). Parallel sequencing analyses throughout a custom panel covering genes implicated in hereditary spastic paraparesis and clinical exome sequencing detected no clinically relevant variants compatible with a dominant or recessive inheritance pattern. SNP array detected a 30 kb microdeletion (Fig. 2A), segregating from her asymptomatic mother, in the 14q31.3 region that includes part of the GALC gene. Based on this finding, GALC activity was assessed on both patient’s leukocytes and skin fibroblasts, providing evidence of significantly reduced values (1.2 nmol/mg, [n.v. 7–34] and 2 nmol/mg [n.v. 5–60], respectively). qRT-PCR analysis consistently showed a reduction of GALC mRNA expression levels of about 80% in patient 1 and 70% in patient 2 compared with those of controls, thus suggesting the presence of a second hit in trans involving the gene (Fig. 2B). RT-PCR was then performed on RNA isolated from cultured fibroblasts to amplify the full-length GALC transcript (NM_000153; coding sequence: 2058 nt), documenting a single fragment of approximately 2000 bp in healthy control subjects and the presence of a second larger band of 2300 bp in both patients (Fig. 2C) with slightly higher expression in patient 1 than in patient 2 (Fig. 2D). Sanger sequencing allowed the characterization of this second cDNA product resulting from an aberrantly spliced GALC transcript, in which a deep intronic region of about 300 bp (“cryptic exon”) was retained downstream exon 14, causing a shift of the reading frame resulting in the incorporation of a divergent 41 nucleotide-long terminus and premature termination (Fig. 2E). Sequencing analysis of the genomic region surrounding the intronic portion incorporated in the transcript allowed us to identify the variant c.1670+2353G>A in a heterozygous state (Fig. 2F). This variant was located 20 bp upstream the retained cryptic exon, suggesting a role in the aberrant processing of the transcript. Segregation analysis confirmed the paternal origin of the variant. At last evaluation at age 11 years, she was able to speak fluently and to walk with spastic gait aided by ankle-foot orthosis for medium-short distances. The WISC-IV revealed a total intelligent quotient (IQ) of 82 and Vineland Adaptive Behavior Scale 2nd edition revealed an IQ of adaptive level of 90 (25th percentile). She underwent matched unrelated donor hematopoietic stem cells transplantation (HSCT) few months ago and follow-up is ongoing in order to adequately monitor the outcome.
Mild T2-weighted hyperintensity in the posterior periventricular white matter and centrum semiovale of non-specific significance at age 18 months (thin arrows in A and B). Progressive worsening of supratentorial white matter hyperintensity is shown, with involvement the splenium of the corpus callosum (thick arrow in E), the bilateral deep white matter at level of corona radiata, centrum semiovale and periventricular regions (thin arrows) at age 9 (D, E) and 10 (G, H) and without cerebellum involvement (C, F, I) or cortical atrophy. White matter lesions appeared hypointense in the T1-weighted images, suggesting a demyelinating leukodystrophy (not shown).
A Image from SNP-array analysis: Chromosome 14 ideogram (left) and the enlargement of the deleted area (yellow box on the right), which partially overlaps the GALC gene. B Quantitative analysis of GALC mRNA expression shows a substantial (80% and 70%) reduction in patient 1 (Pt1) and 2 (Pt2), respectively, compared to controls (Ctrls). Data are expressed as GALC/GAPDH mRNA ratio. C RT-PCR of full-length GALC mRNA shows the presence of a 2000-bp band in two healthy controls (Ctrl1 and Ctrl2), and an additional product of 2300 bp both patients; 1 kb ladder is also indicated (M). Schematic diagram of the aberrant transcript processing causing retention of a portion of intron 14. Exons and cryptic exon are shown in rectangle and dashed rectangle, respectively. Red star indicates the position of the new stop site. D Quantification of the two bands intensities (mut: aberrant transcript; wt: canonical transcript, in C) using ImageJ normalized against the fraction of the total signal (tot). Data are expressed as mut/tot and wt/tot relative ratio. E Organization of the relevant GALC gene portion. Intronic sequences are in lower case letters, coding sequences are in upper case letters. The cryptic exon is labeled by the yellow highlighting. Arrowheads indicate the position of the heterozygous variant 20 bp upstream the beginning of the cryptic exon (c.1670+2353G>A) and the new introduced stop codon, respectively. F Sanger sequencing shows the transmission of the paternal intronic variant in both affected members.
The younger brother is a 6-year-old boy with normal psycho-motor development. He started to manifest a burning sensation of the lower limbs at the age of 3 years. Neurological examination showed mild clumsiness and reduction of deep tendon reflexes at lower limbs. NCS disclosed demyelinating sensory-motor neuropathy and brain MRI revealed very mild hyperintensity of the cerebellar hilus of dentate nuclei on T2-weighted images (Fig. 3A–C). EEG gave normal findings, as well as neuropsycological test (total IQ of 111 at WPPSI-III scale). GALC enzyme activity on leukocytes resulted 0.6 nmol/mg and plasma HexSph was in normal range. Genotyping confirmed the compound heterozygosity for the two GALC variants. A further brain MRI at the age of 5 years was unchanged (not shown). At last neurological evaluation, he was able to speak with mild rhinolalia and mild expressive language disorder; he was able to walk unaided without limitations for his age, although he showed progressive difficulties in running, jumping on one foot, walking in tandem or on heels. Babinski’s sign was present and deep tendon reflex were weak at lower limbs. Screening for HSCT has been started.
Discussion
Early-infantile KD is characterized by normal development in the first few months followed by rapid severe neurologic deterioration with extreme irritability and crying, feeding difficulties, severe spasticity, developmental regression, seizures, blindness, hearing loss, and peripheral neuropathy. Average age of death is 24 months (range: 8 months to 9 years) [1, 3]. Late-onset KD is much more heterogeneous in disease presentation, severity, and course: manifestations range from slow development or regression randomly associated with spasticity, seizures, vision loss, and peripheral neuropathy in late-infantile (onset 12–36 months) to gait abnormalities (e.g., slowly progressive spastic paraparesis, ataxia) in adolescent/adult clinical variants [1, 3]. Early-infantile KD has a more diffuse cerebellar and deep cerebral WM disorder, late-infantile cases show a crossover pattern with less or absent cerebellar involvement and adolescent/adult patients have isolated corticospinal tract involvement [4]. This last pattern has also been reported in one late-infantile [5] and few late-onset KD patients [6,7,8]. Genotype–phenotype correlations indicate that early-infantile KD results from severe GALC deficiency, which is usually associated with homozygosity for the GALC 30 kb deletion or a loss-of-function variant (i.e., frameshift, nonsense, missense substitutions affecting the catalytic activity of the enzyme), or compound heterozygosity for these “severe” variants. The 30 kb deletion is estimated to represent approximately 45% of the pathogenic alleles in populations of European descent, and it is therefore referred as the “common” GALC deletion [1]. Differently, patients with late-onset phenotypes are generally compound heterozygotes for variants including some missense mutations (e.g., p.G57S, p.T112A, p.D187V, p.G286D, p.P318R, p.L634S) or the common deletion. Notwithstanding such genotype–phenotype correlation, same mutations (e.g., p.T529M and p.Y567S) have been associated with both severe and mild phenotypes [9]. Here, we described two siblings with biallelic GALC pathogenic variants manifesting divergent and atypical clinical and neuroimaging phenotypes. The older sister presented with early-onset spastic paraparesis and subclinical neuropathy whose onset was set in the second year of life, thus falling into the “late-infantile” form [3]. A 10-year follow-up indicates a slowly progressive course without involvement of cognition or vision, which is unusual considering age at disease onset [3]. The younger brother has mild ataxic features associated with neuropathy whose onset was set at age 3, thus being recognized as juvenile KD. Brain MRI pattern in the sister seems more similar to the corticospinal tracts involvement seen in adolescent/juvenile or adult KD cases, and currently is very mild in the brother.
Galactosylsphingosine, also known as psychosine, is a well-known biomarker for early-onset form and its measurement in dried blood spots is improving the newborn screening programs for KD; nevertheless, lower or normal values of psychosine may be present in late-onset phenotype [10, 11]. In our patients, we determined the levels of HexSph (galactosylsphingosine + glucosylsphingosine) by liquid chromatography coupled with tandem mass spectrometry, a validate method for simultaneous quantification of several lysosphingolipids [12]. The finding of normal levels of HexSph emphasized the challenging diagnosis of atypical phenotypes as well as late onset KD.
With regard to genotype, to the best of our knowledge no literature data are available on deep intronic GALC variants. Presence of intronic mutation was suggested by low enzyme activity in spite of atypical clinical and neuroimaging features. Phenotypic variability observed in this family resembles that reported in others (i.e., co-existence in the same family of infantile and late-onset forms) [13,14,15,16,17,18] suggesting that unknown modifying factors exist and that prediction of genotype–phenotype correlation may be challenging in KD. There were no clear environmental factors or other diseases (e.g. infections) that might be taken into account to justify such a phenotypic divergence between the two siblings. One possible explanation could be related to a different efficiency in the cryptic splice site. In fact, RT-PCR indicated that only a portion of the mutated allele is undergoing alternative splicing and that the amount of alternative transcript present in patient 2 is less than observed in patient 1, leading us to speculate that this mechanism might explain the milder phenotype observed in patient 2. The atypical genotype and phenotype of this family underlie that there are still missing pieces in the complex puzzle of KD. To date, HSCT is the only effective treatment for KD. Long-term outcome studies in both early- and late-infantile KD demonstrated that timing of HSCT positively influences efficacy [19]. HSCT outcome data are very scarce in juvenile or adult-onset forms [20] and no data are available on “atypical” KD cases like ours. Similar studies describing the long-term outcome after HSCT are needed.
Data availability
Additional data are available from the corresponding author on reasonable request.
References
Orsini JJ, Escolar ML, Wasserstein MP, Caggana M. Krabbe disease. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews®. 2000. [Updated 11 Oct 2018].
Wenger DA, Rafi MA, Luzi P. Molecular genetics of Krabbe disease (globoid cell leukodystrophy): diagnostic and clinical implications. Hum Mutat. 1997;10:268–79.
Bascou N, DeRenzo A, Poe MD, Escolar ML. A prospective natural history study of Krabbe disease in a patient cohort with onset between 6 months and 3 years of life. Orphanet J Rare Dis. 2018;13:126.
Abdelhalim AN, Alberico RA, Barczykowski AL, Duffner PK. Patterns of magnetic resonance imaging abnormalities in symptomatic patients with Krabbe disease correspond to phenotype. Pediatr Neurol. 2014;50:127–34.
Yoshimura A, Kibe T, Irahara K, Sakai N, Yokochi K. Predominant corticospinal tract involvement in a late infant with Krabbe disease. Jpn Clin Med. 2016;7:23–26.
Sehgal R, Sharma S, Sankhyan N, Kumar A, Gulati S. Selective corticospinal tract involvement in late-onset Krabbe disease. Neurology. 2011;77:e20.
Kamate M, Hattiholi V. Predominant corticospinal tract involvement in early-onset Krabbe disease. Pediatr Neurol. 2011;44:155–6.
Nicita F, Graziola F, Vigevano F, Bertini E, Capuano A. An unusual case of late-infantile onset Krabbe disease with selective bilateral corticospinal tract involvement, peripheral demyelinating neuropathy, and mild phenotype. Acta Neurol Belg. 2019;119:619–20.
Madsen AMH, Wibrand F, Lund AM, Ek J, Dunø M, Østergaard E. Genotype and phenotype classification of 29 patients affected by Krabbe disease. JIMD. 2019;46:35–45.
Guenzel AJ, Turgeon CT, Nickander KK, White AL, Peck DS, Pino GB, et al. The critical role of psychosine in screening, diagnosis, and monitoring of Krabbe disease. Genet Med. 2020;22:1108–18.
Corre CS, Matern D, Pellegrino JE, Saavedra-Matiz CA, Orsini JJ, Thompson-Stone R. Low psychosine in Krabbe disease with onset in late infancy: a case report. Int J Neonatal Screen. 2021;7:28.
Polo G, Burlina AP, Kolamunnage TB, Zampieri M, Dionisi-Vici C, Strisciuglio P, et al. Diagnosis of sphingolipidoses: a new simultaneous measurement of lysosphingolipids by LC-MS/MS. Clin Chem Lab Med. 2017;55:403–14.
Fiumara A, Barone R, Arena A, Filocamo M, Lissens W, Pavone L, et al. Krabbe leukodystrophy in a selected population with high rate of late onset forms: longer sur- vival linked to c.121G>A (p.Gly41Ser) mutation. Clin Genet. 2011;80:452–8.
Lyon G, Hagberg B, Evrard P, Allaire C, Pavone L, Vanier M. Symptomatology of late onset Krabbe’s leukodystrophy: the European experience. Dev Neurosci. 1991;13:240–4.
Kolodny EH, Raghavan S, Krivit W. Late-onset Krabbe disease (globoid cell leukodystrophy): clinical and biochemical features of 15 cases. Dev Neurosci. 1991;13:232–9.
Phelps M, Aicardi J, Vanier MT. Late onset Krabbe’s leukodystrophy: a report of four cases. J Neurol Neurosurg Psychiatry. 1991;54:293–6.
Turazzini M, Beltramello A, Bassi R, Del Colle R, Silvestri M. Adult onset Krabbe’s leukodystrophy: a report of 2 cases. Acta Neurol Scand. 1997;96:413–5.
Verdru P, Lammens M, Dom R, Van Elsen A, Carton H. Globoid cell leukodystrophy: a family with both late-infantile and adult type. Neurology. 1991;41:1382–4.
Yoon IC, Bascou NA, Poe MD, Szabolcs P, Escolar ML. Long-term neurodevelopmental outcomes of hematopoietic stem cell transplantation for late-infantile Krabbe disease. Blood. 2021;137:1719–30.
Sharp ME, Laule C, Nantel S, Mädler B, Aul RB, Yip S, et al. Stem cell transplantation for adult-onset krabbe disease: report of a case. JIMD Rep. 2013;10:57–59.
Acknowledgements
The parents are fully acknowledged for their perseverance and patience in both diagnostic and therapeutic phases of their nice sons. FN and EB are members of the European Reference Network for Rare Neurological Diseases – Project ID No 739510.
Funding
The authors report no targeted funding.
Author information
Authors and Affiliations
Contributions
The corresponding author has had full access to the data in the study and final responsibility for the decision to submit for publication. All contributing authors have met the following criteria: conceived and/or designed the work that led to the submission, acquired data, and/or played an important role in interpreting the results; drafted or revised the manuscript; approved the final version; agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. Parents signed consent forms for genetic analyses, participation to this study, and for data publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nicita, F., Stregapede, F., Deodato, F. et al. “Atypical” Krabbe disease in two siblings harboring biallelic GALC mutations including a deep intronic variant. Eur J Hum Genet 30, 984–988 (2022). https://doi.org/10.1038/s41431-022-01111-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-022-01111-z
This article is cited by
-
Exome sequencing—one test to rule them all?
European Journal of Human Genetics (2022)