Abstract
Speech and language impairments are commonly reported in DYRK1A syndrome. Yet, speech and language abilities have not been systematically examined in a prospective cohort study. Speech, language, social behaviour, feeding, and non-verbal communication skills were assessed using standardised tools. The broader health and medical phenotype was documented using caregiver questionnaires, interviews and confirmation with medical records. 38 individuals with DYRK1A syndrome (23 male, median age 8 years 3 months, range 1 year 7 months to 25 years) were recruited. Moderate to severe intellectual disability (ID), autism spectrum disorder (ASD), vision, motor and feeding impairments were common, alongside epilepsy in a third of cases. Speech and language was disordered in all participants. Many acquired some degree of verbal communication, yet few (8/38) developed sufficient oral language skills to rely solely on verbal communication. Speech was characterised by severe apraxia and dysarthria in verbal participants, resulting in markedly poor intelligibility. Those with limited verbal language (30/38) used a combination of sign and graphic augmentative and alternative communication (AAC) systems. Language skills were low across expressive, receptive, and written domains. Most had impaired social behaviours (25/29). Restricted and repetitive interests were most impaired, whilst social motivation was a relative strength. Few individuals with DYRK1A syndrome use verbal speech as their sole means of communication, and hence, all individuals need early access to tailored, graphic AAC systems to support their communication. For those who develop verbal speech, targeted therapy for apraxia and dysarthria should be considered to improve intelligibility and, consequently, communication autonomy.
Similar content being viewed by others
Introduction
Dual specificity tyrosine phosphorylation regulated kinase 1 A, DYRK1A, plays a significant role in brain development and regulating cell proliferation, including shaping the brain and monitoring the structure of neuronal components [1, 2]. DYRK1A is a protein kinase found in the ‘Down syndrome region’ of chromosome 21, that is critical for nervous system development [3,4,5].
Haploinsufficiency of DYRK1A causes DYRK1A syndrome (OMIM 614104); a rare condition with a recognisable but heterogeneous phenotype, including a spectrum from mild to severe intellectual disability (ID), speech and language delays, epilepsy, microcephaly, delayed growth, autism, feeding difficulties, facial gestalt, and vision defects [4, 6,7,8,9,10,11,12]. DYRK1A syndrome constitutes 0.1–0.5% of individuals with ID and/or autism spectrum disorder (ASD) [6].
Speech and language disorders are acknowledged as a core component of DYRK1A syndrome: in a review of 51 previously published and 10 novel DYRK1A cases, Earl et al. (2017) identified that 100% of participants had a language and/or speech impairment [7]. Across the literature, communication issues have been reported as speech and language ‘delays’ or minimally verbal presentations [4, 6,7,8]; however, these reports have been descriptive in nature, without use of standardised clinical protocols or prospective assessments. Hence, despite communication impairment apparently being universal in individuals with DYRK1A variants, there is no deep phenotyping delineating the specific clinical speech and language diagnoses implicated in the condition.
A comprehensive characterization of the speech and language phenotype of DYRK1A syndrome is required to guide clinical intervention and support our understanding of DYRK1A’s role in communication development. Here we provide the first detailed characterisation of speech and language abilities in children with DYRK1A syndrome in the context of the broader health and neurodevelopmental phenotype.
Methods
Participants
Inclusion criteria were a pathogenic loss-of-function variant in DYRK1A and age over 6 months. Genetic reports were provided by families to confirm the molecular diagnosis (Table 1). All but two participants were diagnosed by a range of next generation sequencing assays, including whole genome sequencing, exome sequencing and gene panel testing, undertaken in either a clinical or research setting. The exceptions were participant 19, who was diagnosed by karyotype and participant 20 who was diagnosed by SNP microarray. Exclusion criteria were the presence of other pathogenic variants in addition to DYRK1A. Participants were recruited from advertisements through DYRK1A support groups and through contacting clinical genetic colleagues to highlight the study. Ethics approval was obtained from the Royal Children’s Hospital, Melbourne, Human Research Ethics Committee (HREC 37353 A). Participants’ caregivers provided informed electronic consent to participate in the study. Participants genotype and phenotype information was added to the Decipher database (https://decipher.sanger.ac.uk/).
Health and development
We utilised our previously validated approach of online standardised parent report questionnaires and telehealth assessment. An extensive, 23-page established questionnaire collated health and medical information including developmental history, performance in activities of daily living and psychomotor skills [13, 14]. This questionnaire has been translated across languages including English, Dutch, French, German, Portuguese, Spanish and Italian. Questionnaire responses were confirmed by relevant health and medical reports uploaded to a secure portal by families, e.g., magnetic resonance imaging, electroencephalogram, or cognitive assessment reports.
Telehealth appointments were only conducted with participants with English-speaking caregivers whose caregivers spoke English (31/38) (Table 2). When telehealth appointments were not possible, families provided videos of their children communicating to help verify the health and medical survey results. In addition, English-speaking families of children who were minimally verbal also provided further video example evidence, beyond the brief telehealth assessment session, of the child’s communication abilities, e.g., more examples of their child using Augmentative and Alternative Communication (AAC) systems in real world settings.
Feeding
The Child Oral and Motor Proficiency Scale (ChOMPS) is a validated caregiver questionnaire for children aged 6 months to 7 years [15]. This measure assesses the coordinated movements of oral structures that are required for eating and drinking.
Adaptive behaviour and language
Caregivers completed the Vineland Adaptive Behaviour Scales (VABS-III) as a questionnaire [16, 17]. This tool provides standardised scores for the domains of communication, socialisation, self-care and activities of daily living and motor skills. These standard scores combined to give an overall score of adaptive behaviour. The mean difference between participant’s scores on communication subdomains, expressive and receptive language, was tested using a paired t test.
Non-verbal and social communication
We divided participants into three groups based on verbal language skills, with participants grouped as minimally verbal (defined here as <30 spoken words) [5], using single words and short phrases (SWSP, > 30 spoken words, combining words in short phrases), or using conversational speech (engaging in conversation using speech).
The Inventory of Potential Communicative Acts (IPCA) [18] was completed by caregivers of participants who were minimally verbal. This assessment investigates informal and idiosyncratic forms of communication, such as facial expression, body movement, vocalisations, and gesture. A range of communication functions are also assessed, including social conventions such as saying hello and goodbye, protesting and requesting.
The Social Responsiveness Scale-2 (SRS-2) [19] is a standardised 65-item caregiver questionnaire with three forms: preschool (3–4.5 years), school age (4.5–18 years) and adult (19 years+). The SRS-2 measures autism characteristics, including social awareness, social cognition, social communication, social motivation and restricted interests and repetitive behaviour. These areas are compatible with the DSM-5 diagnostic criteria for ASD [20]. A paired t test was also used to highlight any significant differences between these areas.
Speech
Perceptual speech assessment was used to diagnose motor speech disorders of dysarthria and childhood apraxia of speech (CAS), with verbal participants via telehealth assessment. Stimuli of conversational speech, the Phonology subtest of the Diagnostic Evaluation of Articulation and Phonology (DEAP) [21], a sustained vowel and a diadochokinetic speech task (e.g., say ‘pataka’) were elicited to enable speech ratings. Dysarthria is a neuromuscular execution disorder that affects one or more of the speech subsystems including respiration, phonation, articulation, resonance, or prosody [22]. Dysarthria was rated using the Mayo Clinic dysarthria classification system rating scale [13, 23]. CAS was diagnosed based on the presence of three core features: (i) inconsistency of speech across productions; (ii) disrupted and prolonged co-articulatory transitions and (iii) prosodic errors as defined by ASHA [24]. To rate CAS, we used a previously published protocol [13], validated across several populations to date [14, 25, 26]. Articulation and phonological disorders were identified using the DEAP Phonology subtest [21]. An oral motor systematic protocol [27] was utilised to investigate oral structure and function, using speech and non-speech motor tasks. The Intelligibility in Context Scale [28] was administered as a survey to provide a standardised rating of how easily the child can be understood by familiar listeners to complete strangers. A paired t test was used to assess the mean difference in participants’ intelligibility between familiar and unfamiliar listeners.
Results
Participants
We recruited 38 participants with confirmed pathogenic DYRK1A variants (n = 38, M = 23 F = 15), with a median age of 8 years 3 months (range: 1 year 7 months to 25 years). Of the 38 participants, 36 were novel and 2 were previously published (ID 19; participant 1 in the first study to delineate the clinical features of DYRK1A syndrome Møller et al., 2008 [29]; ID 12, participant 2 in Luco et al., 2016 [30]). Five participants (ID 1, 2, 10, 11, 31) had participated in autism research studies [31, 32] (not yet published). Participants were from the United States [16], the United Kingdom [5], Australia [4], Germany [2], Netherlands [2], Italy [2], Canada [2], Brasil [1], Mexico [1], France [1], Denmark [1], Portugal [1].
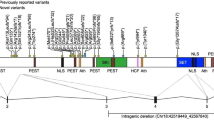
The average age at diagnosis was 7 years 6 months old. Both sets of parental DNA samples were not available for two participants (ID 4 and 30). However, in all other participants inheritance of the DYRK1A variant was heterozygous, de novo (Table 1). Participants had frameshift 32%, nonsense 42%, splice site 13%, and missense variants 8%. One participant had a balanced translocation (ID 19) and another participant had a 2 Mb deletion (ID 20). Three participants with missense variants, as in previous studies, appeared to have similar phenotypes to individuals with truncating variants, translocations, and deletions [33]. Participant 4 had a missense variant of unknown significance. This individual was included as missense variants in the catalytic domain of DYRK1A have been described as pathogenic previously in the literature [33].
Health and development
All participants had broad ranging developmental features across speech and language, feeding and drinking, self-care and daily living, and motor skills (Figs. 1, 2). Most had received support from occupational therapists (33/38) and physiotherapists (33/38) for fine and gross motor skill development (Table 1). In comparison to other domains, motor skills appeared to be a relative strength (Fig. 1). Yet all caregivers noted that all participants still found gross motor tasks (such as riding a bike), more challenging than same-aged peers. Likewise, participants who were walking also had gait impairments (25/37).
Graph showing the number of participants (n = 15, aged 6 months to 7 years) who scored beneath the 5th percentile, between the 5th and 10th percentile and above the 10th percentile on the Child Oral and Motor Proficiency Scale’s subscales. Percentiles are from normative data of same aged peers [12].
A history of ear infections was common (20/38) and only one individual had moderate hearing loss (due to a homozygous pathogenic variant of GJB2, ID 29). Vision problems were prevalent (31/38) including myopia (15/31), strabismus (15/31), hypermetropia (9/31), astigmatism (6/31), optic nerve hypoplasia (5/31), photophobia (2/31) and nystagmus (2/31). Over half the group wore glasses (21/38).
Dysmorphic facial features were seen in all participants including microcephaly (33/38) and retro/micrognathia (15/38). Other shared facial features were ear anomalies (12/38), narrow mouth/thin lips (12/38), broad nasal bridge (8/38), deep-set eyes (6/38) high arched palate (5/38), and short philtrum (4/38). One individual had a diagnosis of submucous cleft palate (ID 22). Other physical features were long fingers and toes (5/38) and pectus excavatum (3/38). Most participants who were old enough to have most of their teeth had dental anomalies (13/31), including frequent dental caries (5/13), complex orthodontics (5/13), excess teeth (3/13) and overcrowding (2/13). Gastrointestinal issues, such as constipation (16/38), reflux (3/38) and gastroparesis (2/38) were noted. Most participants had undergone some type of surgery (26/38). These surgeries were largely for vision impairments (9/38), gastrointestinal tract (e.g., biopsies, tube placement, 9/38), ears (e.g., grommets, 7/38), urogenital conditions (undescended testes, hypospadias, testicular torsion, phimosis) (5/38), hernias (7/38), adenoidectomies (7/38) and tonsillectomies (3/38), and musculoskeletal abnormalities [foot surgeries, scoliosis (5/38)]. 12/30 participants were reported to have poor thermoregulation, such as being unable to sweat or easily becoming too hot or cold. 11/38 participants had current or previous dermatitis, and 11/38 participants had allergies (4/11 had dairy allergies). A few participants had endocrine and metabolic problems (3/38), such as high levels of triglycerides, hypothyroidism, and hypoglycaemia. 9/38 participants had cardiac defects, including atrial and ventricular septal defects (3/9), cardiovascular malformation (2/9), sub-aortic stenosis (2/9), postural orthostatic tachycardia syndrome (1/9), aberrant subclavian artery (1/9), aortic insufficiency (1/9) and hypertrabeculated left ventricle (1/9).
Mild to severe ID was present in all participants who had completed cognitive testing (28/38) (Table 1). For the ten who had not yet completed testing, this was due to a lack of parent or clinician referral/feeling that formal testing to receive a diagnosis was not warranted. ASD was diagnosed in 20/38 of participants. Attention deficit hyperactive disorder (10/38) and behavioural problems were observed (12/38). Behavioural problems were generally described as: aggressiveness (5/12), anxiousness (4/12), restricted interests (5/12), repetitive behaviour (4/12), obsessions (4/12), self-harm (4/12), poor attention (3/12), and hyperactivity (2/12). Seventeen participants had seizures and 16/17 were confirmed to have epilepsy. All 17 participants with seizures were receiving pharmaceutical treatment. A further 8 participants had a history of febrile seizures. For those that had undergone magnetic resonance imaging (MRI) or computerised tomography (CT scan) (n = 36), 27/36 had abnormalities present, including cerebellar atrophy, enlarged ventricles, general reduced volume, and incomplete myelination (Supplementary Table 2). Over half the cohort had had sleep disturbances (23/38), including difficulty falling asleep (13/38) or staying asleep (15/38), waking early (5/38) and central sleep apnoea (2/38).
Feeding
Almost all participants had a history of feeding or swallowing impairment (35/38). Participants frequently struggled with sucking and swallowing in infancy and had a nasogastric (NGT) or gastrostomy tube (PEG/G-tubes) in situ as an infant (16/38). For four participants, feeding support with a G-tube continued into childhood (ID 6, 14, 21, 30). Almost all had notable feeding difficulties, i.e., for overall motor abilities for feeding skills, measured by the ChOMPS, 12 participants were in the bottom 5th, and two individuals were at the 5th to 10th percentile, for their age. Other examples of feeding difficulties included over stuffing their mouth (n = 3), pocketing food in mouth (n = 3), difficulty moving bolus around the mouth (n = 5), likely contributed to by oral praxis difficulties and rotary chewing impairment. Basic movement patterns, such as sitting upright, were strengths relative to other skills, such as complex movement pattern skills (e.g., using a fork or licking food off the upper lip) (Fig. 2). More than half of the participants 8 years and older (10/19) still had feeding or swallowing problems. Drooling was less common than feeding difficulties, though many participants had a history of drooling (15/38), which remained persistent in most (8/15). Many participants also had feeding difficulties due to oral aversion (10/38) and 15/38 took nutritional supplements due to a limited diet.
Adaptive behaviour and language
All individuals had seen a speech therapist, and 30 individuals were currently accessing speech therapy services. Caregivers reported that speech therapy goals focussed on receptive language skills (e.g., following instructions), social communication skills (e.g., communicating and playing with others), verbal speech production (e.g., speech sound production), and expressive language skills (e.g., extending utterance length). 18/38 participants were minimally verbal (<30 words), 12/38 used SWSP (>30 words, combining words), and 8/38 had conversational speech (Table 3). All participants with conversational speech were older than the cohort’s median age, bar one (ID 18). The oral language skills amongst participants with conversational speech was varied. Some participants required support to engage in conversation (e.g., prompting to answer questions; ID 1, 15) whilst others did so independently (ID 2, 16, 18, 25, 34). Of the 13 participants who were combining words, this usually occurred after 4 years of age (11/13). Minimally verbal and SWSP participants used AAC methods to support their communication, in the form of graphic AAC (e.g., communication devices, speech generating devices) and sign language. All individuals that were reported to use sign had less than 15 signs that they used consistently and independently. In the first 2 years of life, gesture and sign was used by 89% of participants, and only one individual used graphic AAC. However, as participants grew up and their communication needs augmented, the prevalence of sign decreased. Sign was used by 75% of participants between 3–5 years old (n = 36), 72% between 6–10 years old (n = 22), 50% between 11–15 years old (n = 14), and 43% 16 years old and over (n = 7). Conversely, the use of graphic AAC mostly increased to supplement verbal communication. AAC was used by 39% of participants between 3–5 years old (n = 36), 45% between 6–10 years old (n = 22), 50% between 11–15 years old (n = 14), and 43% 16 years old and over (n = 7).
Language skills, as measured by the VABS-III, were low (scaled score <9) across receptive, expressive, and written subdomains (Table 3). There was no significant difference between expressive and receptive language (n = 33, p > 0.05, p = 0.42) (Table 3). The cohort averages across all VABS-III domains were low (<70, mean = 100, SD = 15) (Fig. 1), across communication skills (mean standard score = 49.1), daily-living skills (mean = 51.0), social skills (mean = 50.6) and motor skills (mean = 59.0), as measured by the VABS-III (Fig. 1). The average overall score, the adaptive behaviour composite (ABC), was also low (mean = 51.5). VABS-III scores were unavailable for 6 participants, 5 because the assessment was unavailable in their language, and 1 because the assessment was not completed.
In terms of genotype-phenotype associations statistical comparisons across groups were not possible given the small sample size across genotypes (i.e., 3 missense, 5 splice site variants). Yet, boxplot descriptive comparisons revealed that each genotypic subgroup was represented by individuals with a range of language abilities. No group appeared to be better or worse than others in terms of the standardised scores on the VABS-III.
Non-verbal and social communication
Of the 20 participants assessed with the IPCA, 50% used a sign for ‘more’; however, for all other communicative functions sign was used by <20% of participants (Table 4). IPCA results highlighted that most participants (n = 20) exhibited communicative functions that were socially motivated, such as greeting (100%), farewelling (85%), and seeking comfort (85%) (Table 4). Challenging behaviours (such as damaging items, tantrum, or self-injury) and stereotypic behaviours (such as arm flapping and head rocking) were often used as a response when a participant did not like something (Table 4). The IPCA illustrated that communicating specific messages was difficult across the cohort. For example, 70% of the 20 participants could not ask to go to the toilet, 65% could not ask for clarification and 55% could not ask for information (Table 4).
Almost all participants assessed by the SRS-2 (25/29) had problems with social behaviours across all subscales associated with ASD (Fig. 3). More than half (15/29) of assessed participants fell within the severe range for autistic behaviours and only 4 participants were within normal limits for total score, as assessed by the SRS-2 (Table 3). For these participants social cognition (e.g., the ability to interpret social cues) was a strength for some (ID 8 & 34), whilst social motivation (e.g., motivation to engage with others) (ID 2) and social communication (e.g., expressive communication aspect of social behaviour) were strengths for others (ID 23). Across the cohort, social motivation was a strength relative to restrictive and repetitive behaviours (Fig. 3, mean = 60, SD = 10). This contrast between social motivation (mean = 67.4) and restricted and repetitive behaviours (mean = 75.8) was significant across the cohort (p < 0.05).
Higher scores indicate more autism characteristics, (mean = 60, standard deviation = 10). A T score ≤59 indicates social behaviour within normal limits, 60–65 mild difficulty, 66–75 moderate difficulty, ≥76 severe difficulty. Social awareness (mean = 73.3), social cognition (mean = 70.9), social communication (mean = 72.4), social motivation (mean = 62.4), restricted interests and repetitive behaviour (mean = 75.8).
Speech
Of those verbal children assessed for speech, motor speech disorders were common with CAS in 17/18 and dysarthria in 16/18 (14/18 had both dysarthria and CAS). For individuals with CAS, the most common speech features were: groping during speech, 11/17; compromised syllable integrity, 13/17; frequent sound omissions, 10/17; vowel errors, 10/17; syllable segregation, 9/17; impaired achievement of initial articulatory placements, 9/17; and increased errors with word length and complexity, 9/17. Only one participant was receiving a specific speech therapy programme targeted for CAS. Dysarthria was typically characterised by impairments affecting pitch, resonance, and respiration (volume and voice quality), prosody and articulation (Fig. 4). All participants also had phonological and articulation disorders, ranging from mild to severe. During oral motor assessment 8/18 participants were noted to have limited upper lip movement and could not perform oral motor tasks that involved rounding their lips. Poor coordination of the tongue and limited range of tongue movement was evident for many participants (12/18).
Number of participants (16/18) who exhibited specific dysarthric features rated on the Mayo Clinic dysarthria classification system [22].
Across the cohort, intelligibility to familiar listeners (mean = 3.7) was significantly better than intelligibility to unfamiliar listeners (mean = 2.2. On a scale of 1, never understood, to 5, always understood, n = 38, p < 0.05). To familiar listeners, such as caregivers, 10% of participants were never understood, 3% rarely, 10% sometimes, 61% usually, and 16% always understood. To unfamiliar listeners, 29% were never understood, 34% rarely, 29% sometimes, 8% usually, and no participants were always understood by unfamiliar listeners.
Discussion
Here, we described the first systematic characterisation of speech and language in DYRK1A syndrome. To date, communication abilities in DYRK1A syndrome have been non-specifically categorised as a speech and/or language delay. The term speech or language delay is a misnomer because presumably most children do not ‘catch up’ as the term delay implies, but rather have persistent speech and language impairments. Whilst speech and language abilities were varied amongst the cohort, all had significant communication deficits.
Speech and language disorders were ubiquitous, regardless of the type or frequency of other conditions, such as ID, ASD or epilepsy. Most had acquired some verbal communication; however, few developed oral language skills strong enough to rely solely on this method. Language skills were low across expressive, receptive, and written abilities. Contrary to previous clinical observation reports, there was no marked difference between average receptive and expressive language skills [8]. Expressive language skills can appear poorer than receptive language skills in the presence of significant speech sound disorders, as previously noted in relation to SETBP1 haploinsufficiency disorder [14] and FOXP1-related disorders [25]. Only standardised testing can definitively test for perceived discrepancies. Some participants also had stronger oral language skills than previously reported [12]. Most of these participants (7/8) were older than the median age of our cohort and had protracted communication milestones. This may suggest that speech and language skills may continue to improve in some individuals into adolescence. The motor speech disorders of CAS and dysarthria are identified as a common feature of DYRK1A syndrome and if not systematically assessed may impact phenotyping of communication skills. Assessment can also pave the way for application of better targeted speech therapies.
Sign and graphic AAC were used by most participants who were minimally verbal or used single words or short phrases. Motor skills, although a relative strength, were impaired across the cohort. Impaired motor skills and notable vision deficits can greatly impact the ability of an individual to learn sign [34]. Sign was frequently taught between birth and 2 years, however participants usually adopted graphic AAC systems to meet their communication needs as they grew older, possibly due to visual and motor deficits. The small number of participants who acquired verbal conversational skills still reached communication milestones much later than their peers. In these instances, use of graphic AAC may be helpful in the early years to provide a robust method of communication while verbal skills are still developing or as a backup in times of communication breakdown.
CAS features and dysarthria greatly impacted the intelligibility of verbal participants. These speech features indicated impaired motor speech programming, causing disordered organisation, planning and execution of speech. Across the cohort, dysarthria affected all speech sub-systems; respiration and phonation (volume and voice quality), resonance, prosody, and articulation (Fig. 4). Yet, no participants had received specific therapy for dysarthria. Whilst research of developmental dysarthria treatments is limited, there is evidence for approaches that target specific speech sub-systems to improve overall intelligibility [35]. There is more robust RCT evidence for motor programming approaches to treat CAS [36], although only one individual was receiving treatment using a specific motor speech approach. Speech and language features should not be disregarded as merely symptoms of co-morbid neurodevelopmental conditions, and targeted therapies should still be provided despite level of cognitive ability. Similarly, feeding difficulties were also common in infancy and chronic for many individuals. Further work is required to better delineate the core contributing factors to the ongoing feeding issues and to lead to better targeted therapies to improve feeding outcomes [37]. It is essential that specialised speech, language and feeding support is provided to improve outcomes for participants and their families.
All participants either had a diagnosis of an ID or, if they had not undergone cognitive assessment, had global developmental delay. A limitation of this study, and many other reverse phenotyping studies, was that many participants had not received a cognitive assessment, despite being old enough (>2 years old). Additionally, those who were assessed, were not examined with the same assessment battery, though this was unavoidable due to diverse cultural and linguistic backgrounds of the participants. Comprehensive cognitive assessment of all participants would allow for personalised intervention tailored to an individual’s cognitive profile and further support our understanding of the cognitive implications of DYRK1A syndrome.
The SRS-2 and IPCA showed that most participants were socially motivated, having an average social motivation close to within normal limits and frequently engaging in social conventions, respectively. A more nuanced assessment of ASD behaviours and related strengths would aid tailored intervention that could utilise social communication strengths, such as social motivation, to support deficits in other social domains. The SRS-2 identified that 10 participants who did not have an ASD diagnosis had significant autistic behaviours and 7/10 fell in the moderate-severe range of autistic behaviours. Only 4 participants fell within normal limits for their social communication skills, and these individuals had varying oral language skills, but had receptive and expressive language skills higher than the group average. Without a formal diagnosis, individuals with DYRK1A syndrome may miss out on receiving therapy that supports autistic behaviours and learning skills, when this could be of benefit. It can often be difficult to assess ASD in the presence of moderate-severe ID [38], so a detailed assessment of behaviours and comorbidities is important. Cognitive and ASD assessment could improve the quality of intervention provided by therapists and clinicians and enhance our clinical understanding of DYRK1A syndrome.
Conclusion
This study provides further information on the clinical phenotype of DYRK1A syndrome. Speech and language disorders, alongside cognitive impairment, and ASD, are the predominant features of DYRK1A syndrome. Speech and language impairments were heterogenous across the cohort. Few individuals with DYRK1A syndrome use verbal speech as their sole means of communication, and hence, all individuals need early access to tailored, graphic AAC systems to support their communication abilities. For those who develop verbal speech, targeted therapy for apraxia and dysarthria should be considered to improve intelligibility and communication autonomy.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available because families did not consent to this, but are available from the corresponding author on reasonable request and if families provide consent.
References
Guedj F, Pereira PL, Najas S, Barallobre M-J, Chabert C, Souchet B, et al. DYRK1A: a master regulatory protein controlling brain growth. Neurobiol Dis. 2012;46:190–203.
Lin YC, Frei JA, Kilander MB, Shen W, Blatt GJ. A subset of autism-associated genes regulate the structural stability of neurons. Front Cell Neurosci. 2016;17:263.
Arranzranz J, Balducci E, Arató K, Sánchez-Elexpuru G, Najas S, Parras A, et al. Impaired development of neocortical circuits contributes to the neurological alterations in DYRK1A haploinsufficiency syndrome. Neurobiol Dis. 2019;127:210–22.
Ji J, Lee H, Argiropoulos B, Dorrani N, Mann J, Martinez-Agosto JA, et al. DYRK1A haploinsufficiency causes a new recognizable syndrome with microcephaly, intellectual disability, speech impairment, and distinct facies. Eur J Hum Genet: EJHG. 2015;23:1473–81.
Brignell A, Chenausky KV, Song H, Zhu J, Suo C, Morgan AT. Communication interventions for autism spectrum disorder in minimally verbal children, Cochrane Database Syst Rev. 2018;11:CD012324.
van Bonn Bon BW, Coe BP, Bernier R, Green C, Gerdts J, Witherspoon K, et al. Disruptive de novo mutations of DYRK1A lead to a syndromic form of autism and ID. Mol Psychiatry. 2016;21:126–32.
Earlrl RK, Turner TN, Mefford HC, Hudac CM, Gerdts J, Eichler EE, et al. Clinical phenotype of ASD-associated DYRK1A haploinsufficiency. Mol Autism. 2017;8:1–15.
Meissner LE, Macnamara EF, D’Souza P, Yang J, Vezina G, Network UD, et al. DYRK1A pathogenic Var two patients syndromic Intellect Disabil A Rev Lit. 2020;8:e1544.
Murray CR, Abel SN, McClure MB, Foster J. Novel causative variants in DYRK1A, KARS, and KAT6A associated with intellectual disability and additional phenotypic features. J Pediatr Genet. 2017;6:77–83.
Qiao F, Shao B, Wang C, Wang Y, Zhou R, Liu G, et al. A de novo mutation in DYRK1A causes syndromic intellectual disability: a Chinese case report. Front Genet. 2019;10:1194.
Valetto A, Orsini A, Bertini V, Toschi B, Bonuccelli A, Simi F, et al. Molecular cytogenetic characterization of an interstitial deletion of chromosome 21 (21q22. 13q22. 3) in a patient with dysmorphic features, intellectual disability and severe generalized epilepsy. Eur J Med Genet. 2012;55:362–6.
Courraud J, Chater-Diehl E, Durand B, Vincent M, del Mar Muniz Moreno M, Boujelbene I, et al. Integrative approach to interpret DYRK1A variants, leading to a frequent neurodevelopmental disorder. Genet Med. 2021;23:2150–9.
Mei C, Fedorenko E, Amor DJ, Boys A, Hoeflin C, Carew P, et al. Deep phenotyping of speech and language skills in individuals with 16p11. 2 deletion. Eur J Hum Genet: EJHG. 2018;26:676–86.
Morganorgan A, Braden R, Wong M, Colin E, Amor D, Liégeois F, et al. Speech and language deficits are central to SETBP1 haploinsufficiency disorder. Eur J Hum Genet: EJHG. 2021;29:1–10.
Pados BF, Thoyre SM, Park J. Age‐based norm‐reference values for the Child Oral and Motor Proficiency Scale. Acta Paediatrica. 2018;107:1427–32.
Sparrow S, Cicchetti D, Saulnier CJCP, MN: American Guidance Service. Vineland adaptive behavior scales–third edition. 2016.
Sparrow SS, Cicchetti DV, Balla DA. Vineland adaptive behavior scales Vineland-II: Survey forms manual: Pearson Minneapolis, MN; 2005.
Sigafoos J, Woodyatt G, Keen D, Tait K, Tucker M, Roberts-Pennel D. The inventory of potential communicative acts. Enhancing everyday communication for children with disabilities. Paul H Brookes Publishing; 2006, p. 137–50.
Constantino JN, Gruber CP. Social responsiveness scale: SRS-2: Western Psychological Services Torrance, CA; 2012.
American Psychiatric Association DS, American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American psychiatric association; 2013.
Dodd B, Zhu H, Crosbie S, Holm A, Ozanne A. Diagnostic evaluation of articulation and phonology (DEAP). London: The Psychological Corporation; 2002.
Duffy, JR. Motor speech disorders: substrates, differential diagnosis and management. St. Louis: MO: Mosby; 2013.
Morgan AT, Masterton R, Pigdon L, Connelly A, Liegeois FJJB. Functional magnetic resonance imaging of chronic dysarthric speech after childhood brain injury: reliance on a left-hemisphere compensatory network. Brain: J Neurol. 2013;136:646–57.
American Speech-Language Hearing Association. Childhood apraxia of speech [Internet]. Rockville, MD: ASHA; 2022. Available from: https://www.ash.org/public/speech/disorders/childhood-apraxia-of-speech/ (accessed 16 March 2022).
Braden RO, Amor DJ, Fisher SE, Mei C, Myers CT, Mefford H, et al. Severe speech impairment is a distinguishing feature of FOXP1‐related disorder. Dev Med Child Neurol. 2021;63:1417–26.
Bradenraden RO, Boyce JO, Stutterd CA, Pope K, Goel H, Leventer RJ, et al. Speech, Language, and Oromotor Skills in Patients With Polymicrogyria. Neurology. 2021;96:e1898–e912.
Robbins J, Klee TJJoS, Disorders H. Clin Assess oropharyngeal Mot Dev Young- Child. 1987;52:271–7.
McLeod S, Crowe K, Shahaeian AJL. Intelligibility in Context Scale: Normative and validation data for English-speaking preschoolers. Lang, Speech, Hearing Serv Sch. 2015;46:266–76.
Møllerøller RS, Kübart S, Hoeltzenbein M, Heye B, Vogel I, Hansen CP, et al. Truncation of the Down syndrome candidate gene DYRK1A in two unrelated patients with microcephaly. Am J Hum Genet. 2008;82:1165–70.
Luco SM, Pohl D, Sell E, Wagner JD, Dyment DA, Daoud HJBmg. Case report of novel DYRK1A mutations in 2 individuals with syndromic intellectual disability and a review of the literature. BMC Med Genet. 2016;17:1–8.
University of Washington. TIGER study [Internet]. Seattle, WA: University of Washington Autism Centre; 2022. Available from: https://depts.washington.edu/uwautism/research-projects/tiger-study/ (accessed 16 March 2022).
Simon’s Foundation. Simon’s searchlight [Internet]. New York City, NY: Simon’s Foundation; 2022. Available from: https://www.simonssearchlight.org/ (accessed 16 March 2022).
Widowati EW, Ernst S, Hausmann R, Müller-Newen G, Becker WJBo. Functional characterization of DYRK1A missense variants associated with a syndromic form of intellectual deficiency and autism. Biol Open. 2018;7:bio032862.
Aydin O, Diken IHJE, Ti Autism, Disabilities D. Stud comparing augmentative alternative Commun Syst (AAC) Appl Individ autism Spectr Disord: A Syst Rev meta-Anal. 2020;55:119–41.
Pennington L, Parker NK, Kelly H, Miller N. Speech therapy for children with dysarthria acquired before three years of age. Cochrane Database Syst Rev. 2016;7:CD006937.
Morgan AT, Murray E, Liegeois FJ. Interventions for childhood apraxia of speech. Cochrane Database Syst Rev. 2018;5:CD006278.
Morgan AT, Dodrill P, Ward EC. Interventions for oropharyngeal dysphagia in children with neurological impairment. Cochrane Database Syst Rev. 2012;10:CD009456.
Thurm A, Farmer C, Salzman E, Lord C, Bishop SJFip. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526.
Acknowledgements
Our sincere thanks to all the children, young adults and their families who took part in this project. A special thanks to the DYRK1A society for their assistance with recruitment and support of the project.
Funding
Funding was provided by National Health and Medical Research Council (NHMRC) Practitioner Fellowship #1105008 (AM); NHMRC Investigator Grant #1195955. NHMRC Centre of Research Excellence in Speech and Language Neurobiology #1116976 (ATM, DJA). This work was supported by the Victorian Government’s Operational Infrastructure Support Program.
Author information
Authors and Affiliations
Contributions
LDM: generated data, analysed data, interpreted data, wrote manuscript. ROB: generated data, analysed data, interpreted data, wrote manuscript. DJA: analysed data, interpreted data. AB: analysed data, interpreted data. BWMVB: designed and conceptualised study, wrote manuscript. ATM: designed and conceptualised study, directed project, interpreted data, wrote manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethics approval was obtained from the Royal Children’s Hospital, Melbourne, Human Research Ethics Committee (HREC 37353A). Participants’ caregivers provided informed electronic consent to participate in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Morison, L.D., Braden, R.O., Amor, D.J. et al. Social motivation a relative strength in DYRK1A syndrome on a background of significant speech and language impairments. Eur J Hum Genet 30, 800–811 (2022). https://doi.org/10.1038/s41431-022-01079-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-022-01079-w
This article is cited by
-
Shared and divergent mental health characteristics of ADNP-, CHD8- and DYRK1A-related neurodevelopmental conditions
Journal of Neurodevelopmental Disorders (2024)
-
CDK13-related disorder: a deep characterization of speech and language abilities and addition of 33 novel cases
European Journal of Human Genetics (2023)
-
Expanding the speech and language phenotype in Koolen-de Vries syndrome: late onset and periodic stuttering a novel feature
European Journal of Human Genetics (2023)
-
Characterizing Sensory Phenotypes of Subgroups with a Known Genetic Etiology Pertaining to Diagnoses of Autism Spectrum Disorder and Intellectual Disability
Journal of Autism and Developmental Disorders (2023)
-
Clinical genomics testing: mainstreaming and globalising
European Journal of Human Genetics (2022)