Abstract
Multi-locus Inherited Neoplasia Allele Syndrome (MINAS) refers to individuals with germline pathogenic variants in two or more cancer susceptibility genes(CSGs). With increased use of exome/genome sequencing it would be predicted that detection of MINAS would become more frequent. Here we review recent progress in knowledge of MINAS. A systematic literature search for reports of individuals with germline pathogenic variants in 2 or more of 94 CSGs was performed. In addition, participants with multiple primary tumours who underwent genome sequencing as part of the Rare Disease arm of the UK 100,000 Genomes Project were interrogated to detect additional cases. We identified 385 MINAS cases (211 reported in the last 5 years, 6 from 100,000 genomes participants). Most (287/385) cases contained at least one pathogenic variant in either BRCA1 or BRCA2. 108/385 MINAS cases had multiple primary tumours at presentation and a subset of cases presented unusual multiple tumour phenotypes. We conclude that, as predicted, increasing numbers of individuals with MINAS are being have been reported but, except for individuals with BRCA1/BRCA2 MINAS, individual CSG combinations are generally rare. In many cases it appears that the clinical phenotype is that which would be expected from the effects of the constituent CSG variants acting independently. However, in some instances the presence of unusual tumour phenotypes and/or multiple primary tumours suggests that there may be complex interactions between the relevant MINAS CSGs. Systematic reporting of MINAS cases in a MINAS database (e.g. https://databases.lovd.nl/shared/diseases/04296) will facilitate more accurate prognostic predictions for specific CSG combinations.
Similar content being viewed by others
Introduction
Though the development and progression of cancer are primarily driven by the acquisition of somatic genetic and epigenetic events that promote oncogenesis (driver mutations), in a subset of cases, cancer initiation is a consequence of a germline pathogenic variant in a high or moderate penetrance cancer susceptibility gene (CSG) [1, 2]. The proportion of a particular human cancer associated with a germline CSG pathogenic variant differs by site and tumour type being very high in some (e.g. wild-type gastrointestinal stromal tumours, paraganglioma) and low in others (e.g. lung cancer) [3,4,5,6,7]. Similarly, the number of CSGs that can predispose to a specific tumour type is highly variable with some only associated with a single CSG and others with many (e.g. haemangioblastoma and breast cancer respectively). Consequently, the role of, and approach to germline genetic testing in patient management varies such that for some tumour types only a fraction of cases may be tested whilst for others genetic testing can be indicated in the majority of cases. In addition, relevant testing may target a single or numerous CSGs. As advances in genomic technology have reduced the cost of genetic testing, increasing numbers of CSGs have been characterised and the tumour types associated with individual CSGs have expanded. These developments have been associated with a clear trend towards more testing with larger gene panels for many tumour types.
The incidence of pathogenic variants in CSGs in most populations is very low but in some ancestral groups, founder mutations may be as frequent as 1 in 100 individuals (e.g. BRCA1 pathogenic variants in the Ashkenazi Jewish population) [8,9,10,11]. Hence, the odds of an individual having a pathogenic variant in more than one CSG would be predicted to be remote. Nevertheless, as genetic testing expands and gene testing panels become more encompassing, increasing numbers of individuals who harbour pathogenic variants in two or more CSGs are being detected. This phenomenon, labelled MINAS (multilocus inherited neoplasia allele syndrome) was reviewed five years ago [12] and here we revisit the topic to review current knowledge on the occurrence, nature and cancer phenotypes associated with MINAS and to evaluate whether combinations of CSG mutations appear additive or synergistic with regard to cancer risks.
Frequency of MINAS
Previously it was predicted the frequency of MINAS would increase as genetic testing of CSGs expanded [12]. To test this hypothesis a literature review for cases of MINAS using similar methodology to that adopted by Whitworth et al. [12] was performed. Additionally, the AND operator followed by the terms ‘germline mutation’ OR ‘germline’ OR ‘germ-line’ OR ‘double heterozygosity’ OR ‘double heterozygote’ OR ‘genetic predisposition’ OR ‘inherited mutation’ OR ‘MINAS’ OR ‘multilocus inherited neoplasia’ AND ‘cancer’ were used to limit the search to return a smaller number of entries by filtering out superfluous literature (see supplementary data for full details of search strategy). After candidate articles were identified, the pathogenicity of the described CSG variants was assessed to identify pathogenic or likely pathogenic (P/LP) variants (ClinVar \(\ge\)1* P/LP classification or classified as P/LP by American College of Clinical Genetics Guidelines). In addition to searching the published literature, cases of MINAS included in the UK 100,000 genomes data set [13] were sought among participants who were recruited to the Rare Disease arm of the study with a phenotype of ‘tumour predisposition syndromes’ and ‘multiple primary tumours’ and were considered to have MINAS if they were found to have predicted truncating variants in two CSGs (see supplementary material).
In addition to the 89 cases reported by Whitworth et al. [12], 290 further MINAS cases from the literature and 6 from 100,000 Genomes Project data [13] were identified to provide a final total of 385 individuals with MINAS (see Supplementary Table 3 and additional details in Supplementary Table 4). The 385 cases were plotted according to the year of description (the six cases from 100,000 genomes project were included under 2020) and this revealed a progressive increase in the cumulative total with an apparent acceleration since 2016 (see Fig. 1).
Genetic architecture of MINAS
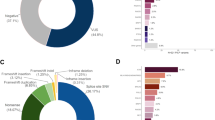
Reviewing the genetic architecture of the 385 individuals (current cohort) with 430 unique P/LP variants in 63 CSGs, a BRCA1 and/or BRCA2 variant was present in 78.5% of cases (287/385) (see Fig. 2). The current cohort was subdivided into those described previously in Whitworth et al (2016) (historical subgroup, n = 89) and those identified more recently (recent subgroup) and comparison of the two subgroups showed that the proportion of BRCA1/BRCA2 containing MINAS reports had increased slightly (75.3% (67/89) and 79% (220/296) respectively) (see Table 1). Comparing the MINAS-associated CSGs in the ‘historical subgroup’ and the ‘recent subgroup, revealed 45 CSGs not previously reported in MINAS cases that were present in the recent subgroup (including ATM, CHEK2, FH) and variants in these CSGs were present in 29% (113/385) of all cases (Table 1). Though in the historical subgroup, P/LP variants in FLCN were present in 6.7% (6/89) of cases, there were no additional cases in the ‘recent subgroup’ (0/296). The genes that were most frequently reported in recent subgroup MINAS reports, after BRCA1 and BRCA2, included CHEK2, ATM and FANCM, which would be consistent with these ‘newer’ breast CSGs increasingly being included in gene testing panels for breast/ovarian cancer susceptibility [14,15,16]. It should be noted that not all of the genes that were included in our MINAS searching strategy (e.g. BLM, ERCC3, ERCC5, RECQL4, XPA, FANCA, FANCC) are known to be disease causing in a monoallelic P/LP variant state. However, on balance, we considered that these genes (marked with superscript a in Table 1) should be included in a MINAS cohort as there is the possibility that they might act as modifiers in the presence of CSG P/LP variant that was associated with a phenotype in the heterozygous state (particularly if both CSGs were functionally related e.g. implicated in DNA repair pathways). Excluding MINAS cases that included these CSGs would reduce the number MINAS cases by 32 (10 from the ‘historical subgroup’ and 22 in the ‘recent subgroup’).
Phenotypic consequences of MINAS
As discussed previously [12], it could be proposed that the adverse phenotypic consequences of MINAS could be additive (i.e. the observed cancer risks reflect those of each the relevant CSGs independent of the presence of the other) or synergistic (i.e. some CSG combinations could result in notably more severe phenotypes such as earlier ages at onset or the occurrence of tumour types that are atypical for the relevant CSGs (theoretically MINAS might also be associated with protective effects (e.g. through synthetic lethality) but this would be probably require analysis of healthy control cohorts rather than patients tested through diagnostic laboratories). To review the evidence for additive/synergistic effects, we subdivided the 385 MINAS cases in the current cohort into those with a BRCA1/BRCA2 MINAS combination (n = 206) and those with other combinations of CSGs and then examined possible evidence for additive/synergistic interactions. However, for assessing the occurrence of tumour types that are atypical for the relevant CSGs, for non-BRCA1/BRCA2 MINAS combinations, the number of instances of specific CSG combinations was generally very small. 108/385 (28%) MINAS cases had multiple primary tumours at presentation. Among the 108 cases, 2 (1.9%) had an unknown number of multiple primaries, 75 (69%) had had two primary tumours, 18 (17%) had had three and 13 (12%) had four or more. The most common multiple primary tumour combinations were Breast-Ovarian, with 33 cases, Breast-Breast with 24 cases and Colon-Colon with 6 cases.
Phenotypic associations of non-BRCA1/BRCA2 MINAS
In the MINAS historical subgroup it was estimated that 14.6% of patients (13/89) had at least one tumour type that was not typical of the relevant CSGs (e.g. renal clear cell carcinoma in a patient with variants in both BRCA1 and MLH1) [12]. However, in the recent cohort an atypical tumour phenotype was present in 15.8% (12/76) of non-BRCA1/BRCA2 MINAS cases (though not all studies presented individual patient-level data). Details of patients with multiple primary tumours are shown in Supplementary Table 5 and those with atypical tumour phenotypes are highlighted. Four examples of atypical MINAS phenotypes were (see Supplementary Tables 3/4 for further details) were:
-
1.
A woman diagnosed with breast cancer and Waldenstrom’s disease aged 58 years with pathogenic variants in BRCA1 and BLM. (From Sokolenko et al., [17]).
-
2.
A woman diagnosed with breast cancer, melanoma and colorectal cancer with a pathogenic variant in FANCC and P/LP variant in TYR (Stolarova et al., [18]).
-
3.
A woman diagnosed with lobular breast cancer at 51 years of age, followed by follicular adenoma and thyroid micropapillary carcinoma at 52 years with pathogenic variants in PMS2 and CDH1 (Njoroge et al., [19]).
-
4.
A woman diagnosed with cutaneous leiomyomas at 40 years followed by colorectal polyposis at 52 years was found to be heterozygous for P/LP variants in FH and BARD1 (Stradella et al., [20]).
In these cases, Waldenstrom disease, colorectal cancer, thyroid carcinoma and colorectal polyposis occurred despite not being associated with any of the relevant MINAS CSGs [21,22,23,24]. Each of these cases presented with multiple primary tumours and rare CSG combinations so it is not possible to say whether this was a manifestation of synergy between the relevant CSGs or coincidental (and the presence of multiple tumours of unusual types might have been more likely to prompt genetic testing).
Phenotypic associations of BRCA1/BRCA2 MINAS
In a cohort of 32,295 females with BRCA1/BRCA2 P/LP variants, Rebbeck et al. [25] identified 93 women with BRCA1/BRCA2-MINAS and reported that although there was no significant difference in the mean age at breast cancer diagnosis between BRCA1 only pathogenic variant and the BRCA1/BRCA2 MINAS women, there was an earlier age at breast cancer diagnosis (~4.5 years less) and increased incidence of ovarian cancer in women with BRCA1/BRCA2 MINAS compared to BRCA2 pathogenic variant carriers. In addition, BRCA1/BRCA2 MINAS women were significantly more likely than BRCA1 or BRCA2 women to have had breast cancer [25]. In total, our literature review identified 206 cases of BRCA1/BRCA2 MINAS and, after excluding those cases reported by Rebbeck et al. (90), the mean(+SD) age at breast cancer diagnosis was 42.4 (+-10 years, n = 69 compared to 40.4 years in the Rebbeck et al. BRCA1/BRCA2 MINAS cohort and 41.9 (n = 9316) and 45.0 (n = 3370) in their BRCA1-only and BRCA2-only pathogenic variant carriers. These findings were consistent with the assertion in Rebbeck et al. [25] that mean age at breast cancer diagnosis on BRCA1/BRCA2 MINAS is similar to that in BRCA1 pathogenic variant carriers.
Phenotypic associations of MINAS caused by breast cancer predisposition genes
In addition to those cases with BRCA1/BRCA2 MINAS, there were also women with a pathogenic variant in BRCA1 or BRCA2 plus a pathogenic variant in another breast CSG. With the trend towards larger gene panels for breast cancer predisposition testing, increasing numbers of patients will be tested for BRCA1/BRCA2 and moderate risk breast CSGs. Which moderate-risk breast CSGs are tested can vary between centres but a recent work by Dorling et al. recommend a hereditary breast cancer screening panel of ATM, BARD1, BRCA1, BRCA2, CHEK2, PALB2, RAD51C, RAD51D and TP53 would contain the most clinically useful CSGs [26].
Tumour studies and mechanisms of tumourigenesis in MINAS
When multiple primaries occur in association with MINAS and the relevant CSGs have very different tumour associations, it may be straightforward to assign the occurrence of a particular tumour to a specific CSG (notwithstanding the occurrence of atypical tumours as discussed previously). However, when the CSGs have overlapping tumour associations this is more difficult and indeed, the most frequent examples of MINAS involve multiple breast CSGs. In such cases the application of tumour studies might provide insights into whether a single CSG or multiple CSGs are implicated in the occurrence of a tumour. As most CSGs follow a tumour suppressor ‘two hit model’ of tumourigenesis the most readily available strategy for investigating mechanisms of tumourigenesis is tumour loss of heterozygosity (LOH) studies (though LOH may not be observed if the somatic inactivating event is a point mutation or promoter methylation of the wild-type allele) [27, 28]. Previously Rebbeck et al. [25] described LOH analysis from 14 informative cancers from BRCA1/BRCA2 MINAS cases and found LOH at a single locus in four tumours suggesting that in most cases the tumour develop from a second hit at a single CSG and the effects of MINAS are generally additive rather than synergistic.
When considering combinations of CSGs that might result in a synergistic effect on tumour risks, it has been suggested that mutations in CSGs that map to the same chromosome region might have a more adverse effect as LOH causing loss of a single chromosome harbouring the CSG wild type alleles would result in a tumour homozygous null for both CSGs. Other potential adverse combinations might include two CSG oncogenes, a direct relationship between the mechanisms of tumorigenesis of the two mutations (e.g. APC and mismatch repair gene mutations) or the CSG products being in the same cellular pathway. Other tumour profiling strategies that might provide insights into MINAS mechanisms of tumourigenesis include immunohistochemistry for CSG gene products, microsatellite instability testing and cancer mutational signature analysis [29,30,31]. We note that Rebbeck et al. [25] reported differences in oestrogen/progesterone receptor expression status of breast tumours in women with BRCA1/BRCA2 MINAS compared to those with P/LP variants in BRCA1 or BRCA2 only such that MINAS cases were more likely to be oestrogen receptor (ER) and progesterone receptor (PR) positive breast cancers than BRCA1-only cases and less likely to be ER- and PR-positive than in BRCA2-only cases. This places the receptor phenotypes of the MINAS breast cancers intermediate between that of BRCA2 only and BRCA1 only breast cancers, and would be consistent with an independent rather than synergistic effect in BRCA1/BRCA2 MINAS.
Conclusions
Since the term MINAS was coined 5 years ago, there has been increasing awareness of the phenomenon and this is reflected in the increasing numbers of publications. There are some limitations to our analysis that would cause us to underestimate the frequency of MINAS. Firstly, only a fraction of MINAS cases is likely to be included in the published literature. Secondly, in order to reliably compare the frequency of MINAS in the current literature to that in 2016, we did not include recently identified cancer susceptibility genes. Thirdly, the cases reported to date are predominanty based on diagnostic gene-panel data and future studies based on exome or genome sequencing data would likely yield more cases of MINAS. Nevertheless, the availability of the MINAS database (https://databases.lovd.nl/shared/diseases/04296) provides a dynamic resource that enables data on published and unpublished cases to be shared widely. This is particularly important because, apart from BRCA1/BRCA2 MINAS, other CSG MINAS combinations are rare and so clinicians faced with an individual with non-BRCA1/BRCA2 MINAS will often find it very difficult to predict what the implications are for tumour risks in that individual. Though in most cases the evidence would appear to suggest that the effects are likely to be additive, reports of some MINAS cases with atypical tumours are a concern (though these cases may be overrepresented because of ascertainment bias). To provide the best prognostic information for individuals with MINAS, long-term follow up and molecular genetic analysis of any MINAS-related cancers (e.g. for LOH, cancer signature etc.) should be undertaken.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files and in the MINAS database at https://databases.lovd.nl/shared/diseases/04296.
References
Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74.
Hodgson S. Mechanisms of inherited cancer susceptibility. J Zhejiang Univ: Science B. 2008;9:1–4.
Fishbein L, Nathanson KL. Pheochromocytoma and paraganglioma: Understanding the complexities of the genetic background. Cancer Genet. 2012;205:1–11.
Kanwal M, Ding XJ, Cao Y. Familial risk for lung cancer. Oncol Lett. 2017;13:535–42.
Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. GeneReviews®. 1993. http://www.ncbi.nlm.nih.gov/pubmed/20301425.
Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18.
Norton JA, Krampitz G, Jensen RT. Multiple endocrine neoplasia: genetics and clinical management. Surg Oncol Clin North Am. 2015;24:795–832.
Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA. The prevalence of common BRCA 1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet. 1999;64:963–70.
Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, et al. The risk of cancer-associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–8.
Satagopan JM, Offit K, Foulkes W, Robson ME, Wacholder S, Eng CM, et al. The lifetime risks of breast cancer in Ashkenazi Jewish carriers of brca1 and brca2 mutations. Cancer Epidemiol Biomarkers Prevent. 2001;10:467–73.
Struewing JP, Abeliovich D, Peretz T, Avishai N, Kaback MM, Collins FS, et al. The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet. 1995;11:198–200.
Whitworth J, Skytte AB, Sunde L, Lim DH, Arends MJ, Happerfield L, et al. Multilocus inherited neoplasia alleles syndrome. JAMA Oncol. 2016;2:373–9.
Caulfield M, Davies J, Dennys M, Elbahy L, Fowler T, Hill S, et al. The National Genomics Research Library v5.1. Genom Engl. [Internet]. 2020. https://doi.org/10.6084/m9.figshare.4530893.v6.
Bertelsen B, Tuxen IV, Yde CW, Gabrielaite M, Torp MH, Kinalis S, et al. High frequency of pathogenic germline variants within homologous recombination repair in patients with advanced cancer. npj Genome Med. 2019;4:13.
Taylor A, Brady AF, Frayling IM, Hanson H, Tischkowitz M, Turnbull C, et al. Consensus for genes to be included on cancer panel tests offered by UK genetics services: guidelines of the UK Cancer Genetics Group. J Med Genet. 2018;55:372–7.
Vysotskaia V, Kaseniit KE, Bucheit L, Ready K, Price K, Johansen Taber K. Clinical utility of hereditary cancer panel testing: Impact of PALB2, ATM, CHEK2, NBN, BRIP1, RAD51C, and RAD51D results on patient management and adherence to provider recommendations. Cancer. 2020;126:549–58.
Sokolenko AP, Bogdanova N, Kluzniak W, Preobrazhenskaya EV, Kuligina ES, Iyevleva AG, et al. Double heterozygotes among breast cancer patients analyzed for BRCA1, CHEK2, ATM, NBN/NBS1, and BLM germ-line mutations. Breast Cancer Res Treat. 2014;145:553–62.
Stolarova L, Jelinkova S, Storchova R, Machackova E, Zemankova P, Vocka M, et al. Identification of Germline Mutations in Melanoma Patients with Early Onset, Double Primary Tumors, or Family Cancer History by NGS Analysis of 217 Genes. Biomedicines. 2020;8:404.
Njoroge SW, Burgess KR, Cobleigh MA, Alnajar HH, Gattuso P, Usha L. Hereditary diffuse gastric cancer and lynch syndromes in a BRCA1/2 negative breast cancer patient. Breast Cancer Res Treat. 2017;166:315–9.
Stradella A, Del Valle J, Rofes P, Feliubadaló L, Grau Garces È, Velasco À, et al. Does multilocus inherited neoplasia alleles syndrome have severe clinical expression? J Med Genet. 2019;56:521–5.
Monge J, Braggio E, Ansell SM. Genetic factors and pathogenesis of waldenström’s macroglobulinemia. Curr Oncol Rep. 2013;15:450–6.
Yurgelun MB, Kulke MH, Fuchs CS, Allen BA, Uno H, Hornick JL, et al. Cancer susceptibility gene mutations in individuals with colorectal cancer. J Clin Oncol. 2017;35:1086–95.
Bano G, Hodgson S. Diagnosis and management of hereditary thyroid cancer. Recent Results in Cancer Research. 2016;205:29–44.
Talseth-Palmer BA. The genetic basis of colonic adenomatous polyposis syndromes. Hereditary Cancer Clin Pract. 2017;15:5.
Rebbeck TR, Friebel TM, Mitra N, Wan F, Chen S, Andrulis IL, et al. Inheritance of deleterious mutations at both BRCA1 and BRCA2 in an international sample of 32,295 women. Breast Cancer Res. 2016;18:112.
Breast Cancer Association Consortium, Dorling L, Carvalho S, Allen J, González-Neira A, Luccarini C, Wahlström C, et al. Breast Cancer Risk Genes — association analysis in more than 113,000 women. N Engl J Med. 2021;384:428–39.
Baylin SB, Jones PA. Epigenetic determinants of cancer. Cold Spring Harbor Perspect Biol. 2016;8:a019505.
Thiagalingam S, Laken S, Willson JKV, Markowitz SD, Kinzler KW, Vogelstein B, et al. Mechanisms underlying losses of heterozygosity in human colorectal cancers. Proc Natl Acad Sci U.S.A. 2001;98:2698–702.
Kaliyappan K, Palanisamy M, Duraiyan J, Govindarajan R. Applications of immunohistochemistry. J Pharm Bioallied Sci. 2012;4:S307–9.
Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen H-Z, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;1:PO.17.00073.
Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578:94–101.
Acknowledgements
This research was supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014). The University of Cambridge has received salary support (ERM) from the NHS in the East of England through the Clinical Academic Reserve. The views expressed are those of the authors and not necessarily those of the NHS or Department of Health. We acknowledge support from the NIHR UK Rare Genetic Disease Research Consortium. Part of this research was made possible through access to the data and findings generated by the 100,000 Genomes Project. The 100,000 Genomes Project is managed by Genomics England Limited (a wholly-owned company of the Department of Health and Social Care). The 100,000 Genomes Project is funded by the National Institute for Health Research and NHS England. The Wellcome Trust, Cancer Research UK and the Medical Research Council have also funded research infrastructure. The 100,000 Genomes Project uses data provided by patients and collected by the National Health Service as part of their care and support.
Author information
Authors and Affiliations
Consortia
Contributions
AMcG undertook the literature search and data analysis and co-wrote the first draft and approved the final version. JW, AA, and TH analysed data and critically appraised the manuscript and approved the final version. MT critically appraised the manuscript and approved the final version. ERM conceived and supervised the project, interpreted data, co-wrote the first draft, and critically appraised the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Ethics approval
Data were collected from published reports and from the 100,000 genomes project (these data can be accessed by any researcher who is approved to join a GeCIP domain). The Molecular Pathology of Human Genetic Disease study was approved by the South Birmingham Research Ethics Committee.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McGuigan, A., Whitworth, J., Andreou, A. et al. Multilocus Inherited Neoplasia Allele Syndrome (MINAS): an update. Eur J Hum Genet 30, 265–270 (2022). https://doi.org/10.1038/s41431-021-01013-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-021-01013-6
This article is cited by
-
2022: the year that was in the European Journal of Human Genetics
European Journal of Human Genetics (2023)
-
Lynch syndrome: influence of additional susceptibility variants on cancer risk
European Journal of Human Genetics (2023)
-
Good genotype-phenotype relationships in rare disease are hard to find
European Journal of Human Genetics (2022)
-
The simplest explanation does not have to be preferred: co-occurrence of pathogenic variants in cancer-predisposing genes
European Journal of Human Genetics (2022)