Abstract
Tumour genomic profiling (TGP), conducted in search of therapeutics, sometimes reveals potentially pathogenic germline variants as secondary findings (SFs). Physicians involved in TGP are often specialised in oncology and not in clinical genetics. To better utilise SFs, we explored issues physicians have during disclosure and the potential for collaborations with clinical genetics professionals. Semi-structured interviews were conducted with 14 physicians who had experience in handling outpatient TGP at designated core hospitals for cancer genomic medicine in Japan. The data were analysed thematically. The difficulties physicians experienced during informed consent (IC) included educating patients about SFs, providing detailed information on SFs, and explaining the impact of SFs on patients’ family members. When SFs were detected, physicians had reservations regarding the relevance of the disclosure criteria. Confirmatory germline tests were performed using peripheral blood when tumour-only tests detected suspected SFs. Some physicians had reservations about the necessity of confirmatory tests when they did not affect the patients’ treatment options. To encourage patients to receive confirmatory tests, improvements are necessary in the healthcare system, such as insurance reimbursements, education for physicians so that they can provide a better explanation to their patients, and genetic literacy of physicians and patients. The physicians offered insights into the challenges they experienced related to IC, disclosure of SFs, and expectations for active collaborations with clinical genetics professionals. Wider healthcare insurance coverage and better genetic literacy of the population may lead to more patients taking confirmatory tests when SFs are suspected.
Similar content being viewed by others
Introduction
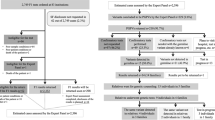
Tumour genomic profiling (TGP) is a comprehensive test for cancer-related gene variants in tumour cells to identify effective cancer therapeutics. The two general types of TGPs are tumour-only sequencing and paired tumour-normal sequencing which uses normal tissues such as peripheral blood to detect germline variants. Both are covered by the National Health Insurance (NHI) of Japan. To be reimbursed by the NHI, the following two steps are required, regardless of which types of TGPs are used; informed consent (IC) needs to be obtained after an explanation that TGPs may detect secondary findings (SFs) which are also called germline findings, and the results need to be directly returned to the patient. Thus, under the NHI scheme, physicians always meet their patients twice, before and after the test. The results of TGPs are returned to patients after annotation by a regularly held intra-institutional molecular tumour board, referred to as Expert Panels (EPs). EP meetings are held at 12 core hospitals for cancer genomic medicine in Japan, which are designated by the Ministry of Health, Labour, and Welfare [1]. EPs consist of multidisciplinary specialists, including oncologists, medical geneticists, genetic counsellors, pathologists, cancer genomics experts, bioinformaticians, and primary physicians [2]. The ability to hold EP meetings is one of the requirements of designated core hospitals.
The purpose of TGPs is primarily to search for pathogenic variants in somatic cells, but they may also reveal pathogenic variants in the germline. These variants are detected in 6.3–15.7% of patients who undergo TGP [3, 4]. For example, 7% of prostate cancer patients who received TGP had results that suggested pathogenic germline variants (PGVs) of BRCA1/2 [4]. These genes are responsible for hereditary breast and ovarian cancer syndromes, which are preventable with effective health management, such as frequent health check-ups and risk-reducing surgeries [5]. Although these findings are unrelated to the original indication for testing (i.e. tumour mutation profiling), they provide useful information for health management and are referred to as SFs. Since SFs are pathogenic variants in the germline, patients may share the same pathogenic variant with their family members. Therefore, informing patients about their SFs is important not only for their health but also for their family members’ health.
Between the two types of TGPs, namely tumour-only sequencing and paired tumour-normal sequencing, there are differences in how the SFs are handled. Whereas tumour-normal paired sequencing detects PGVs, tumour-only sequencing can only detect presumed germline pathogenic variants (PGPVs). To confirm that PGPVs are true PGVs, patients must undergo germline confirmation using peripheral blood through clinical genetics care. Currently, the confirmatory germline test is not reimbursed by the NHI since the concurrent use of insured and uninsured medical treatment is prohibited [6]; therefore, patients who have PGPVs are required to visit the hospital again, separate from their cancer care, to receive a confirmatory germline test.
TGPs usually involve physicians who specialise in cancer treatment, but do not necessarily specialise in clinical genetics. In addition, the main purpose of the TGP is to identify somatic pathological variants in search of therapeutic agents; thus, SFs are not of the highest concern for physicians. This may lead to a lack of awareness regarding SFs in cancer treatment. Both patients and physicians are important stakeholders in TGP [7,8,9]. Previous studies, which focused on patients’ attitudes toward SFs disclosure, revealed that more than half of the patients were willing to have their SFs disclosed because they saw high value in knowledge related to their present or future health [10]. However, few studies have examined physicians’ attitudes and experiences. Therefore, the challenges physicians face when obtaining IC, or when SFs are detected, are unknown. To better utilise SFs, we explored issues physicians have during disclosure, their perspectives toward SFs, and the potential for collaborations with clinical genetics professionals.
Methods
Study design
A qualitative methodology was chosen because it is useful for exploring areas that have not been extensively researched [11]. The Standards for Reporting Qualitative Research (SRQR) checklist was used in this study [12]. This study was approved by the Research Ethics Committee of Kyoto University (R2525).
Recruitment and participants
To recruit study participants, an email was sent by the first author (SS) to physicians who had experience in outpatient TGP at the four designated core hospitals for cancer genomic medicine in Japan. Four hospitals were chosen to geographically represent the nation. Purposive sampling was used to ensure that the participants had experience in handling TGPs and SFs. In the recruitment letter, SS introduced herself as a graduate student studying genetic counselling. To avoid potential biases, we recruited participants from five different medical specialities who did not have a personal relationship with SS.
Data-collection
A consent form and an information sheet were sent to each participant by mail or email. Consent was obtained both in writing and orally before the start of the interview. The information sheet asked for participant demographics, such as years of practice, participant’s sex, how long they have been involved in outpatient TGP, the number of TGPs conducted, and their experience in disclosing SFs to their patients. Semi-structured online interviews were conducted between August and December 2020. All interviews were conducted by the first author (SS). Consent was obtained for video-recording prior to each interview, which were performed using the video conferencing tool Zoom (Zoom Video Communications, Inc., San Jose, CA, USA). The duration of the interview was 54 min on average (range: 25–90 min).
The interview guide consisted of a mixture of open-ended and closed-ended questions (Table 1). This gave structure to the interview process while leaving some flexibility in the choice of wording and sequencing of questions open to the interviewer. Pilot interviews were conducted to improve the interview guidelines. The questions focused on the experiences of participants, reactions of patients (from the perspective of the physician), and the potential for collaborations with clinical genetics professionals. The interview guide was modified throughout the study by including additional lines of questions to ensure that the emerging themes were fully explored.
Data analysis
Recruitment was discontinued once the data were saturated, i.e. when no new themes emerged from the data that were being analysed concurrently with collection. The analysis was initiated after the first interview. Video files were transcribed verbatim and anonymised by SS, who was the only person to have access to the recordings. Transcripts were analysed using inductive thematic analysis [13, 14] because it examines patterns or themes that are present within textual data. First, each transcript was read multiple times to gain familiarity with the data and to identify broad discussion topics related to themes within that thread. Notes were made of the initial findings and observations during this stage. Second, a constant comparison was used to refine and develop these notes by moving back and forth across the dataset comparing threads that discussed specific issues. The responses of the participants in the interviews were coded and initial themes were identified by SS; thereafter, author MI, who had extensive knowledge of qualitative data analysis, reviewed the produced codes and themes to ensure appropriateness. After coding the transcripts from all of the interviews, SS and author TY, who had experience in qualitative study, discussed any discrepancies among codes, subthemes, and themes until a consensus was reached. MAXQDA 2020 software (VERBI Software, Berlin, Germany) was used to facilitate data coding and code categorisation. Representative quotes from the interviews were translated from Japanese to English and are presented to support our findings.
Results
Participant characteristics
Fourteen physicians were recruited, and all of them participated in individual interviews. A total of 71% of participants in this study reported that they had disclosed SFs to their patients (Table 2). The remaining 29% who did not have experience disclosing SFs had seen EP discussions regarding SFs, and one participant had attended a SFs disclosure session.
Theme 1: challenges during the consent process
The responses of the participants to questions in five theme areas are summarised in Table 3. Participants expressed difficulties in helping patients understand SFs because of the patients’ poor health conditions, which led to a compromised understanding of SFs. ‘Different patients have a different level of understanding about what we say. They are sick and feel ill, and therefore, I imagine that they didn’t feel like trying to comprehend topics like [SFs]. Young patients, and those who are doing relatively well on treatment, seem to be able to listen to the explanation.’ (#14) Participants were also aware of their inability to provide detailed information about SFs. The physicians felt that they could not provide patients with sufficient information regarding adequate health management. They highlighted that the key to better understanding was to acknowledge the information needs of the patients. Participants faced challenges explaining the impact of SFs on a patient’s family members. When participants explained the impact of SFs to the patients, they often spoke only about the impact on their children, but not about the potential impact on the patient’s siblings, nieces, and nephews because the participants did not think that SFs were relevant to the extended family members. In addition, the participants recognised the need for patient brochures and other materials to help explain the effects of SFs on their family members. They also expressed a great deal of difficulty in asking about patient family history, which participants needed to better understand the possibility that a hereditary tumour may run in a family. ‘Family history is very important, but it is quite difficult to draw a perfect family tree for physicians specialised in cancer treatment, like me. Drawing a family tree is quite cumbersome.’ (#4) It was also revealed that the depth and scope of the elicited family history details were different among the participants; some asked only about a patient’s family history of tumours, whereas others asked for related family tree history as well.
Theme 2: reactions of participants when SFs were detected
Participants stated that their concerns about detecting SFs decreased with experience on the EP and research analysis. However, they had reservations about the relevance of SF disclosure criteria. They stated that PGPVs should be disclosed based on phenotype rather than allele frequency. Others felt conflicted that only a limited number of genes were disclosed in the whole-exome sequencing because they believed in a patient’s right to know. They also stated that they encountered other physicians who did not follow the SFs disclosure criteria of the institution, and that the disclosure criteria were very restrictive owing to the shortage of genetic counsellors. Participants lacked seriousness in thinking about SFs, as suggested by their comment to the effect that SFs are detected only infrequently. ‘Explaining the result? We only have a few SFs suspect cases. If there are no findings, that’s it. I haven’t put any thought into it because there are only a few cases.’ (#13) They were only disclosing SFs of preventable diseases. The participants said they did not consider PGPVs to be of importance because they were not PGVs and merely indicated uncertain variants. The participants did not pay a lot of attention because their involvement ended with disclosure of the SFs. Participants felt reluctant about explaining to patients whose comprehension was presumed to be compromised. The participants explained that, to avoid unnecessary stress on patients and their families, SFs should be disclosed only to those who understand the uncertain nature of SFs; that is, penetrance is seldom 100%. They also expressed ambivalence about SF disclosure when they put themselves in the patients’ shoes. For example, the participants referred to the mixed feelings that patients must have; they did not fully buy into the benefits of SFs, and they felt that patients should have been informed of germline information much earlier.
Theme 3: participants’ expectations of clinical genetics professionals
The participants wanted better collaborations with clinical genetics professionals. First, participants were eager to know what kind of follow-up was done by clinical genetics professionals. Specifically, they wanted to know (1) whether patients underwent confirmatory germline tests, (2) if they did, the result of the test, and (3) their future health management plan. ‘Feedback is lacking about what happens to the patients after the SFs disclosure. I want to know what happened to my patients after that.’ (#3) Second, participants wanted genetic counsellors to be present during IC so that patients could receive detailed information about the benefits of SFs. Usually, clinical genetics professionals are only present after detecting SFs owing to the lack of workforce, but participants preferred them to be present during IC as well. ‘Ideally, if a genetic counsellor sits with the patients and gives a brief explanation to them about SFs at the IC, the patients could understand SFs much better. We only know which variants cause which diseases and how one gene is associated with a disease very perfunctorily. We are too busy to tell the patients in depth. We are not experts on the matter, either. It would be much better if genetic counsellors could do that for us.’ (#7) Participants expected clinical genetics professionals to have a better knowledge of cancer care and to more actively participate in it. They wanted clinical genetics professionals to understand the health status of their patients. ‘Of course, the primary objective is to choose medical treatments. But if there are colleagues who can support patients when SF results come out, we can have better collaboration. It’d be great if [clinical genetics professionals] were more accessible to our practice as well as to our patients.’ (#8)
Theme 4: reservations about the necessity of the confirmatory germline test
When PGPVs are detected by tumour-only sequencing, confirmatory germline tests are performed using peripheral blood. Participants suggested that confirming SFs were of low priority for patients who already have advanced cancers because preventing additional cancer in the future was not relevant for them. Insured TGPs are conducted only after the completion of standard cancer care and when patients are already in advanced stages of cancer. Participants also added that SFs are not universally beneficial. Participants were more concerned about treatment; therefore, they did not see the importance of confirming SFs. They also remarked that, even though patients should be informed about the confirmatory germline tests, it was up to the patients whether they received the information or not. ‘Those who get TGP covered by insurance are basically with non-curative cancers. What value would PGV give to the patients? Well, for their family members, the story may be a little different. So, if it’s like, “I have a family member who means a lot to me, I’ve got to do this for them”, then I understand. But if the patient doesn’t have any children, or all of the siblings are aged, SFs are less significant. How SFs matter would depend on the patient’s family environment, I think. So, if you are asking if we can raise the overall [confirmatory germline] test ratio, I’m not sure about it when I look at the population profile.’ (#12)
Theme 5: issues surrounding the confirmatory germline test
The participants advocated for a better healthcare system. They referred directly to the necessity of reimbursing confirmatory germline tests to increase access to the tests, making confirmatory germline tests more affordable and convenient. ‘It’s like 30k yen, just to look at one variant. A patient didn’t come back after hearing how much it costs, though I thought he would get tested.’ (#7) ‘The confirmatory test is yet to be reimbursed, so the patient needs to come back to us on a different day, which is another hurdle. This may discourage some patients from getting the test.’ (#10) Currently, insured TGPs are usually conducted only after the completion of standard cancer care. Therefore, they also stated that confirmatory germline tests would be conducted more widely if TGPs were performed earlier in treatment, when patients still have the capacity to pay attention to SFs. Since SFs can benefit not only patients but also their family members, participants emphasised the importance of relaying the information to the patients’ family members. One way to increase family members’ access to genetic counselling (GC) is to conduct it online; confirmatory germline testing is performed through GC. For patients to understand the benefits of the confirmatory germline test, physicians need to be able to describe them adequately. Therefore, participants suggested that there is a need for educating physicians so that they can provide a better explanation to their patients. ‘The patients meet clinical genetics professionals for the first time, while they have been seeing their physicians for years. No matter how many times the clinical genetics professionals talk to the patients about the test, it’s no match for a single word from their attending physician.’ (#6) Participants confessed that they sometimes had a hard time describing what typically happens during GC. To increase physicians’ understanding, it is important to make GC easier for them to visualise. ‘Genetic counsellors start to be involved only after PGPVs are detected; physicians like us do not know enough in depth [about what goes on in GC]. I wonder what is being done; I hope that I can talk to the patients a little better.’ (#10) Participants also pointed out the need for improvements in genetic literacy of both the healthcare professionals and the public, psychological support, and legal protection against discrimination based on genetic conditions. Furthermore, they suggested the need for an increase in resources for clinical genetics professionals through training.
Discussion
This study identified perceptions about SFs of physicians who conduct TGPs, the difficulties they face in handling SFs, and the potential and need for collaborations with clinical genetics professionals. Participants faced challenges in helping patients understand the significance of SFs. In addition, physicians had reservations about the necessity of a confirmatory germline test when PGPVs are detected.
Physicians who handle TGPs felt challenged by the lack of patient understanding of SFs, both at the time of consent and disclosure. Previous research has suggested that patients do not fully understand SFs at the time of consent [15], and this study suggested that this was due to their compromised health condition. Their health condition will likely further deteriorate at the time of disclosure, which makes it even harder for the patients to comprehend the benefits of SFs for themselves and their family members. Almost 65% of cancer patients experience cancer-unique fatigue, which is affected by disease severity and persistent in spite of rest or sleep [16]. For patients to better understand SFs, an explanation has to be given at the time of consent when they are still in better condition.
To improve patients’ understanding of the benefits of SFs, it is important to improve the consent process by providing more detailed information, such as which family members will be affected by SF information and what kind of effect it may have. Previous studies have reported the importance of discussing the expected emotions and subsequent steps of PGPV/PGV detection before testing [17]. One important characteristic of genetics is that a variant is shared with other members of the family [18]. Therefore, it is important to anticipate the impact of SFs on patients’ family members at the time of consent. Through this study, it became clear that to explain the impact on family members effectively, it is crucial to obtain family history information before the test. However, it was suggested that asking for family information is a burden for physicians. Currently, there seems to be no set standard regarding the scope of questions that physicians should ask their patients. Some physicians ask only about the family history of the tumour, while others ask about a broader family history of other diseases as well as their family structure. It is essential to explore effective ways to collect family history and explain the effects on family members based on family history to promote patients’ understanding of the benefits of SFs disclosure.
This study identified that physicians handling TGPs have reservations about SFs disclosure criteria, the importance of SFs disclosure, the significance of disclosure due to the low frequency of PGPV/PGV detection, and the low likelihood of PGPV actually being PGV. In addition, when participants explained the effects of SFs on family members, the focus was mostly on the child and not on the siblings or siblings’ children, suggesting that there is less willingness to explain SFs, especially to patients who do not have a child. Usually, patients will first hear about SFs from the attending physician. For the patient to understand the significance of SFs disclosure, it is necessary for the attending physician to first understand that the finding is beneficial to both the patient and their family members.
Currently, a clinical genetics specialist is usually present only after SFs are detected owing to a shortage of staff. However, the results suggested that physicians in charge want clinical genetics specialists to be present from the time of IC, before providing TGP. According to a previous study, if providers discuss with the patients how others have reacted to SFs and what the patient might do if a SF was discovered, it helped the patients process emotions [17]. Thus, it is preferable to identify the cases in which patients may have difficulties understanding SFs or a highly suspected family history, so that clinical genetics professionals can be present at the time of IC. Cancer genomic medical coordinators assist physicians in the procedural aspect of TGP. They are nurses, pharmacists, or clinical lab technicians [18]. The requirement to become a cancer genomic medical coordinator is to attend a workshop once, as opposed to the two-year-postgraduate education that genetic counsellors need to obtain. Cancer genomic medical coordinators are not equipped to offer relevant information catered to the patient’s individual needs. Thus, it is urgent to increase GC resources. To make the best use of the current GC resources, online access can be of great use, because genetic counsellors are even more scarce in rural areas.
Since patients who undergo TGPs usually have advanced cancer, there is often a limited amount of time left. The physicians in charge wanted clinical genetics professionals to take this into account when patients are considering confirmatory germline tests and requested a concise explanation of SFs in GC.
Paired tumour-normal sequencing and tumour-only sequencing are both covered by the NHI with the same pricing, but confirmatory germline testing in tumour-only sequencing, if done alone, is not reimbursed. The results indicate that if the confirmatory germline tests were also covered by insurance and made available simultaneously with the SFs disclosure, accessibility for patients would increase significantly.
Workshops on clinical genetics for healthcare professionals are useful to enhance their genetic literacy. In such workshops, it is especially important to illustrate how clinical genetics is relevant to them [19]. Knowledge of the susceptibility to hereditary cancer can be beneficial for a better health management of the patients. Healthcare professionals need to be aware that this leads to an empowerment of patients. For higher literacy among the general public, the key is to increase their exposure to adequate information through media and school education. Familiarity with genetics will lower the barriers people feel when they decide whether to take the confirmatory tests. Uneven access to GC is another problem. To meet the growing need for genetic counsellors in the absence of a significant growth in the workforce, online access is needed. Previous studies have found similar levels of patients’ understanding and satisfaction between in-person and online GC [20,21,22,23].
This study had several limitations. First, the participating physicians in this study only worked at designated core hospitals for cancer genomic medicine that have a clinical genetics department to ensure their experience with TGPs and SFs. Therefore, their expectations for clinical genetics professionals may be higher than the actual situation in Japan, and the results may not be generalisable. Second, we asked participants to describe the barriers to performing a confirmatory germline test, but the decision was made by the patients in the end. Therefore, to understand the patients’ point of view, further research on patients who undergo TGP is needed to understand the barriers to confirmatory germline testing.
In conclusion, physicians offered insights into the challenges they experienced related to IC and disclosure of SFs, as well as the necessity for active collaborations with genetics professionals. Wider healthcare insurance coverage and better genetic literacy of the population may lead to more patients undergoing confirmatory germline tests when SFs are suspected.
Data availability statement
The datasets generated during and/or analysed during the current study are not publicly available due to them containing information that could compromise research participant privacy but are available from the corresponding author on reasonable request.
References
Mukai Y, Ueno H. Establishment and implementation of Cancer Genomic Medicine in Japan. Cancer Sci. 2021;112:970–7. https://doi.org/10.1111/cas.14754.
Japan Agency for Medical Research and Development. Proposal concerning the information transmission process in genomic medicine Part 1: Focusing on comprehensive tumor genomic profiling analysis [Revised 2nd edition]. 2019. https://www.amed.go.jp/content/000064662.pdf.
Kou T, Kanai M, Yamamoto Y, Kamada M, Nakatsui M, Sakuma T, et al. Clinical sequencing using a next-generation sequencing-based multiplex gene assay in patients with advanced solid tumors. Cancer Sci.2017;108:1440–6.
Schrader KA, Cheng DT, Joseph V, Prasad M, Walsh M, Zehir A, et al. Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol.2016;2:104–11.
Daly M, Pal T, Berry M, Buys S, Dickson P, Domchek S. et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:77–102. https://doi.org/10.6004/jnccn.2021.0001.
Chino T. An economic analysis of institutions and regulations in the Japanese healthcare system. Gov Audit Rev.2007;14:13–25.
Blanchette PS, Spreafico A, Miller FA, Chan K, Bytautas J, Kang S. et al. Genomic testing in cancer: patient knowledge, attitudes, and expectations. Cancer. 2014;120:3066–73.
Miller FA, Hayeems RZ, Bytautas JP, Bedard PL, Ernst S, Hirte H, et al. Testing personalized medicine: patient and physician expectations of next-generation genomic sequencing in late-stage cancer care. Eur J Hum Genet.2014;22:391–5.
Liang R, Meiser B, Smith S, Kasparian NA, Lewis CR, Chin M, et al. Advanced cancer patients’ attitudes towards, and experiences with, screening for somatic mutations in tumours: a qualitative study. Eur J Cancer Care (Engl). 2017;26:e12600.
Hamilton JG, Shuk E, Genoff Garzon M, Rodríguez VM, Westerman J, Hay JL, et al. Decision-making preferences about secondary germline findings that arise from tumor genomic profiling among patients with advanced cancers. JCO Precis Oncol.2017;1:1–13.
Denzin NK, Lincoln YS. editors. Handbook of qualitative research. CA, USA: Sage Publications, Inc. Thousand Oaks; 1994. p. 643–xii.
O’Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med.2014;89:1245–51.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol.2006;3:77–101.
Braun V, Clarke V. Successful qualitative research: a practical guide for beginners. London: SAGE Publications; 2013.
Downing NR, Williams JK, Daack-Hirsch S, Driessnack M, Simon CM. Genetics specialists’ perspectives on disclosure of genomic incidental findings in the clinical setting. Patient Educ Couns.2013;90:133–8.
Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, et al. Cancer-related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann Oncol.2020;31:713–23.
Rost C, Dent KM, Botkin J, Rothwell E. Experiences and lessons learned by genetic counselors in returning secondary genetic findings to patients. J Genet Couns.2020;29:1234–44.
Naito Y, Aburatani H, Amano T, Baba E, Furukawa T, Hayashida T, et al. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (edition 2.1). Int J Clin Oncol.2021;26:233–83.
Roundtable on Translating Genomic-Based Research for Health, Board on Health Sciences Policy, Institute of Medicine. Improving Genetics Education in Graduate and Continuing Health Professional Education: Workshop Summary. Washington, D.C., USA: National Academies Press; 2015. p. 57–9.
D’Agincourt-Canning L, McGillivray B, Panabaker K, Scott J, Pearn A, Ridge Y, et al. Evaluation of genetic counseling for hereditary cancer by videoconference in British Columbia. B C Med J.2008;50:554–9.
Lea DH, Johnson JL, Ellingwood S, Allan W, Patel A, Smith R. Telegenetics in Maine: Successful clinical and educational service delivery model developed from a 3-year pilot project. Genet Med.2005;7:21–7.
Zilliacus EM, Meiser B, Lobb EA, Kirk J, Warwick L, Tucker K. Women’s experience of telehealth cancer genetic counseling. J Genet Couns.2010;19:463–72.
Zilliacus EM, Meiser B, Lobb EA, Kelly PJ, Barlow-Stewart K, Kirk JA, et al. Are videoconferenced consultations as effective as face-to-face consultations for hereditary breast and ovarian cancer genetic counseling? Genet Med.2011;13:933–41.
Acknowledgements
We would like to thank all physicians who participated in our project.
Funding
This study was supported by the Japan Ministry of Health, Labour and Welfare (grant numbers: 20AD1001 and 19FC1006).
Author information
Authors and Affiliations
Contributions
SS and TY designed the study. SS conducted interviews. SS, TY, and MI analysed the data. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Research Ethics Committee of Kyoto University (R2525).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shimada, S., Yamada, T., Iwakuma, M. et al. Physicians’ perceptions of the factors influencing disclosure of secondary findings in tumour genomic profiling in Japan: a qualitative study. Eur J Hum Genet 30, 88–94 (2022). https://doi.org/10.1038/s41431-021-00944-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-021-00944-4
This article is cited by
-
Management of patients with presumed germline pathogenic variant from tumor-only genomic sequencing: A retrospective analysis at a single facility
Journal of Human Genetics (2023)
-
Challenges of secondary finding disclosure in genomic medicine in rare diseases: A nation-wide survey of Japanese facilities outsourcing comprehensive genetic testing
Journal of Human Genetics (2023)
-
Current status and issues related to secondary findings in the first public insurance covered tumor genomic profiling in Japan: multi-site questionnaire survey
Journal of Human Genetics (2022)