Abstract
SETBP1 haploinsufficiency disorder (MIM#616078) is caused by haploinsufficiency of SETBP1 on chromosome 18q12.3, but there has not yet been any systematic evaluation of the major features of this monogenic syndrome, assessing penetrance and expressivity. We describe the first comprehensive study to delineate the associated clinical phenotype, with findings from 34 individuals, including 24 novel cases, all of whom have a SETBP1 loss-of-function variant or single (coding) gene deletion, confirmed by molecular diagnostics. The most commonly reported clinical features included mild motor developmental delay, speech impairment, intellectual disability, hypotonia, vision impairment, attention/concentration deficits, and hyperactivity. Although there is a mild overlap in certain facial features, the disorder does not lead to a distinctive recognizable facial gestalt. As well as providing insight into the clinical spectrum of SETBP1 haploinsufficiency disorder, this reports puts forward care recommendations for patient management.
Similar content being viewed by others
Introduction
In 2010, we identified de novo gain-of-function variants in SET Binding Protein 1 (SETBP1) as the cause of Schinzel–Giedion syndrome (SGS - MIM #269150) (ref. [1]), a severe multi-system disorder consisting of recognizable facial characteristics, neurological problems (including severe intellectual disability, intractable epilepsy, cerebral blindness, and deafness) and various congenital anomalies (such as heart defects, abnormal genitals, kidney anomalies, and bone abnormalities).
Haploinsufficiency of SETBP1, either caused by a heterozygous gene deletion or loss-of-function (LoF) variant, causes SETBP1 haploinsufficiency disorder currently designated as Mental Retardation Dominant 29 (MIM #616078), which leads to a much less severe phenotype than SGS.
Putative clinical features of SETBP1 haploinsufficiency disorder, including intellectual disability, behavioral problems, and mild dysmorphisms, were first reported in patients with microscopically visible partial deletions of chromosome 18q12 (ref. [2,3,4]). Subsequently, submicroscopic 18q.12 deletions were reported to be associated with a more specific phenotype with expressive speech and language impairments as a prominent feature [5,6,7]. Based on two submicroscopic deletions within chromosome band 18q12.3, the critical region of overlap for this more restricted phenotype was defined and included SETBP1 as the major candidate gene [8, 9].
More recently, whole exome/genome sequencing studies in two cohorts of children with childhood apraxia of speech (CAS) independently suggested haploinsufficiency of the SETBP1 gene as a possible monogenic cause of CAS [10, 11]. Unfortunately, accessible clinical information about individuals with SETBP1 haploinsufficiency disorder based on existing literature is highly limited. Even though 10 cases with variants in SETBP1 have been published in various different studies [10,11,12,13,14], the majority of clinical data are hidden in supplementary files of large cohort studies reporting on the results of novel sequencing techniques. Therefore, a comprehensive clinical characterization of the phenotype of this disorder is still needed. A gene-driven approach, identifying the major features associated with SETBP1 loss-of-function and how they vary between different individuals in a substantive cohort of patients, would represent a valuable advance for the field.
The purpose of this report is to systematically delineate the clinical spectrum of individuals with SETBP1 haploinsufficiency disorder and provide care recommendations for patient management. We present the clinical findings of 24 novel individuals with a SETBP1 loss-of-function variant and integrate information from previously reported individuals, including newly gathered phenotypic findings for three of these cases, to give a fully comprehensive view of this important disorder.
Materials, subjects, and methods
Molecular analyses
Peripheral blood samples were provided in a diagnostic context for the proband, and parents when available. Results originated from diagnostic whole-exome sequencing (WES) or gene panels for intellectual disability or epilepsy (details in Supplementary Table 1). All genetic variants were confirmed by Sanger sequencing. The single gene deletion (individual 21) was diagnosed with array CGH analysis. All variants and phenotypes have been submitted to the LOVD database [15] (https://databases.lovd.nl/shared/variants/SETBP1/unique). The database ID numbers of the variants are listed in supplementary table 1.
Patients
This study analyzed the medical data for patients with a molecularly confirmed diagnosis of SETBP1 haploinsufficiency disorder. The clinical data were obtained through international collaborations involving clinicians from various countries (Supplementary Table 1). Some of these collaborations were established via GeneMatcher [16]. Written and informed consents were obtained from all patients or legal guardians. Consent was also obtained for publication of patient photos, where appropriate.
Only patients with heterozygous loss of function (LoF) variants or heterozygous pure coding SETBP1 gene deletions (not comprising adjacent genes) and no other known clinically relevant variants were included.
17 patients and three previously published cases presented in this study also participated in a separate investigation specifically designed for in-depth assessments of speech and language development (manuscript in progress Morgan et al.).
Results
Molecular results
The different LoF variants and their positions with respect to the SETBP1 protein are summarized in a schematic overview (Fig. 1). Individual 21 was the only novel patient who had a single gene deletion of SETBP1, without encompassing adjacent genes. The other 23 novel patients had a LoF variant, including 10 stop-gain, and 13 frame-shift variants. The variants were distributed through the SETBP1 locus and each is expected to undergo nonsense-mediated decay of the mutant mRNA transcript, yielding LoF and haploinsufficiency. In 21 patients, these variants were confirmed as de novo (91%). In the other three individuals, parental DNA was unavailable. Of these, two were affected sisters (individual three and four) who carried the same variant. The parents passed away and therefore parental testing could not be performed. The parents had been in good health and had normal cognitive function. The two healthy siblings did not carry the variant. These variants are most likely caused by germline mosaicism or low level parental somatic mosaicism.
For cDNA annotation of the variants see supplementary table 1.
Patients
This study analyzed the clinical data for 34 patients with a molecularly confirmed diagnosis of SETBP1 haploinsufficiency disorder. Included were 10 previously reported patients and 24 novel patients. We excluded cases from the literature where clinical data were unavailable, cases with larger CNVs including one or more adjacent genes in addition to SETBP1 and patients with a second molecularly confirmed diagnosis. Patients were diagnosed in eight different countries (Supplementary Table 1). The male:female ratio was 19:15 (56% male). The age at the last examination ranged between 1 and 73 years. An overview of the main clinical features is shown in Table 1, including the numbers and percentages of the individuals that could be analyzed for each given feature and distinguishing between novel cases and those from prior literature. Data on all features for each individual patient is shown in supplementary table 1.
Psychomotor development
In total, 33 of the 34 individuals showed motor developmental delay (97%). Milestones like sitting, crawling, and walking were delayed in most of the individuals (Fig. 2A). The age of sitting unsupported within our study group ranged from 6 to 15 months with a mean of 9.3 months (normative range 4.1–8.4 months [17]). The mean age of crawling was 12.2 months with a range of 9 to 19 months (normative range 5.8–12 months). The mean age of walking unsupported was 19.7 months with a range of 13–36 months (normative range 9–16 months). Two individuals were not yet crawling or walking at the age of 6 months and 14 months, respectively.
A Motor developmental milestones, with green lines highlighting the normal range of the milestone in the general population. B Speech milestones of the verbal individuals. C Growth parameters at birth and at last examination. The mean of each variable is marked with a bold line. OFC occipital frontal circumference.
Individual 10 who is 16 years old used a wheelchair due to exercise intolerance during childhood. Metabolic screening and a muscle biopsy to assess mitochondrial involvement showed isolated decreased ATP production during early childhood but completely normal results at 13 years of age. At 13 years of age she rarely used the wheelchair anymore. She did still show fatigue after intensive days.
Nine individuals have reported that they learned to ride a bike at a mean age of 5 years and 2 months with a range of 4–7 years. Two individuals reported that they could not yet ride a bike at the age of 7 and 8 years, respectively. We did not have data about cycling skills from the remainder of the cohort.
A delay in fine motor skills was reported in 11 individuals (30%).
17 patients and three previously published cases presented in this study also participated in a separate investigation specifically designed for in-depth assessments of current speech and language development (Morgan et al. in progress). Within the current study, we collected retrospective data on this topic. Speech delay was reported in 33 individuals (97%). The mean age of first words was 2 years and 1 month, with a median of 18 months and range of 11 months to 11 years (Fig. 2B). The mean age of the first sentences was 5 years, the median age 4 years with a range of 2–14 years. Four individuals did not speak at the ages of 15 months, 2, 4, and 16 years respectively. In addition, six individuals did not speak short sentences at the ages of 4, 4, 6, 6, 7, and 12 years.
Intellectual disability (ID) of various levels was noted in 23 of 30 patients (77%) who reported data on this topic. There was a broad range of abilities in these individuals. In total, 17% had a normal IQ, 7% had a borderline IQ, 30% showed mild ID, 27% moderate ID, and 20% severe ID. The reason for diagnostic tests in individuals with a normal IQ usually comprised speech delay and/or behavior problems during childhood.
Growth parameters
An overview of the growth parameters is presented in Fig. 2C. Birth country-specific growth charts were used. When these were not available WHO growth percentiles have been used. In general, there are no common growth abnormalities seen across the majority of individuals. Birth weight percentiles ranged between 2 and 80 with an average of 37. Three individuals had a birth weight two SDs below the mean. The weight percentile at the last examination varied between 2 and 99 with a mean of 48. One individual had a current weight of 2 SDs below the mean and another individual had a current weight two SDs above the mean.
Height percentiles ranged between 1 and 99 at birth and 1 and 98 at the last examination. The mean of the height percentiles was 57 at birth and 47 at the last examination. At birth, two patients had a length above two SDs and one patient below two SDs. At the last examination three individuals had a length below two SDs and one individual above two SDs.
At the last examination the OFC of 23 patients was measured. The OFC percentiles ranged between 3 and 98 with a mean of 60. Two individuals had an OFC > P95 and one < P5.
Behavior
Behavior problems of various kinds have been reported in 26 of the 34 patients (76%) (Table 1). The most commonly described issues are attention/concentration deficits in 20 patients (59%) and hyperactivity in 12 cases (35%). Six of these individuals were officially diagnosed with ADHD (18%) and one with ADD. Four patients were officially diagnosed with autism (12%). Other reported behavior problems included anxieties (24%), temper tantrums (24%), aggressive behavior (21%), sleep problems (12%), and self-mutilation (8%). Less frequently reported problems included unrestrained eating behavior and obsessions. Although temper tantrums were noted in eight children, it should be noted that five of these children were aged four years or younger, which is a common age to show such behavior.
Neurology
Hypotonia was a commonly reported neurological feature. In total, 14 patients showed hypotonia (52%), mainly reported in childhood. Individual 1 was the only individual showing hypertonia/spasticity. Seizures were reported in seven of the cases (21%), the majority of which were infantile febrile seizures (in 5/7 cases). One literature case suffered from an unreported form of epilepsy from 3 to 5 years of age requiring medication. Another previously reported individual presented with generalized seizures that started at the age of 22 years [14]. EEG had been performed in eight patients of which two individuals showed diffuse slowing without epileptiform activity. One individual showed interictal epileptiform activity at the right centroparietal lobe during sleep. A cerebral MRI was performed in 18 cases. Two of which showed myelination delay at the ages of 18 and 24 months respectively. The other MRIs showed no abnormalities.
Other clinical features
Vision impairment was present in 14 individuals (48%), with the most common problems being hypermetropia (31%), astigmatism (10%), myopia (10%), and strabismus (14%). Other described vision problems included exophoria (n = 1), amblyopia (n = 1), color blindness (n = 1), and lack of binuclear vision (n = 1).
Hearing impairment was reported in three cases (9%). Individual 10 presented with one-sided conductive 30 dB hearing loss at the age of 9 years after recurrent infections (perforated eardrum). Individual 20 was diagnosed with bilateral asymmetric mild hearing loss (25–39 dB) before the age of 3 years. A 70 dB unilateral hearing loss was reported at the age of 52 years in an individual from the prior literature [14].
Eighteen of the 27 patients reported general health issues. Seven individuals (26%) reported recurrent ear infections, while six (22%) reported sensitive skin or eczema. Allergies to gluten, soy or milk were reported in four patients (15%), two of which had celiac disease. Other described features were feeding problems, asthma, low energy, diarrhea, and reflux presenting at a young age. Muscle biopsies were taken from two individuals to exclude the possibility of a mitochondrial disease.
Dysmorphisms
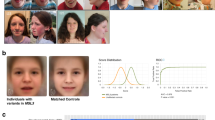
Information about dysmorphisms was reported in 30 individuals of which 28 had facial dysmorphisms (93%) (Supplementary Table 1). In most individuals the facial features were subtle. Common features included ptosis, blepharophimosis, broad nasal bridge, hypertelorism, full nasal tip and high palate. Clinical photographs of a subset of individuals who consented for publication are presented in Fig. 3.
Congenital anomalies
Congenital anomalies were noted for 11 of the 22 individuals (50%) who reported information in this category. Five individuals had ankyloglossia (23%) for which they had frenectomy, while three males had undescended testicles (23% of males who reported on presence or absence of congenital abnormalities). The following congenital anomalies have been reported only once in the individuals studied here: an abnormal vertebra, cleft lip and palate, tracheomalacia, unilateral atrophic kidney, dilated aortic root, facial nerve palsy, and bilateral hip dysplasia. The individual with kidney issues had the same renal phenotype as his brother who did not have SETBP1 haploinsufficiency disorder.
Discussion
This study is the first to focus on describing SETBP1 haploinsufficiency disorder from a genotype-driven perspective, providing a systematic delineation of the associated clinical phenotype. The SET Binding Protein 1 regulates transcription processes by binding to different promotor regions. This protein is highly expressed during brain development. The precise functions and molecular mechanisms of the SETBP1 protein in the brain, and the neuronal pathways that go awry in the associated disorder are largely unknown [18].
The 33 individuals have 29 different de novo variants in SETBP1 (Fig. 1). The majority of individuals carried frameshift (19 variants) or nonsense variants (14 variants), predicted to yield truncation of the encoded protein. These LoF variants were scattered at different points throughout this large gene locus (Fig. 1). Notably, all variants are predicted to be degraded by nonsense-mediated decay according to NMDEscPredictor [19], consistent with haploinsufficiency of SETBP1 protein as an etiological mechanism causing the associated disorder. In all cases where parental testing was possible (88%), de novo occurrence was confirmed. The presence of a sibpair with the same variant warrants attention for the possibility of germline or low-grade somatic mosaicism in one of the parents. In this family, parents had passed away, but the two healthy siblings did not show the variant.
SETBP1 is a protein of 1597 amino acids. None of the variants that we studied are present in the gnomAD database (http://gnomad.broadinstitute.com). In addition, data from the ExAC database (http://exac.broadinstitute.com) indicate that SETBP1 is extremely intolerant for LoF variants (LoF intolerance score of 1.0) (ref. [20]), supporting the pathogenicity of the variants investigated in this cohort. One different truncating variant, NC_000018.10:g.45063631del, that passed the quality filters has been reported in gnomAD. However, this variant is located in the final exon and most likely escapes from nonsense-mediated decay according to NMDEscPredictor [19].
Our analysis shows that the main clinical features of SETBP1 haploinsufficiency disorder include moderate to severe speech impairment (see Morgan et al., in progress), mild motor developmental delay, a wide range of intellectual functioning (from normal IQ to severe ID), hypotonia in childhood and behavior problems. The majority of children do not experience major motor problems at a later age. Although speech impairments are evident in almost all children with this disorder, formal ID is not present in 23% of individuals. Therefore, genetic testing of SETBP1 in children with speech impairment or significant behavior problems who have a normal or borderline IQ may be considered.
The most commonly reported behavioral characteristics were attention deficit (59%) and hyperactivity (35%). Since only 18% of individuals were officially diagnosed with ADHD we suggest that a neuropsychologic assessment at diagnosis would be helpful as a subset of these children will likely meet the criteria for an official ADHD diagnosis and may benefit from tailored guidance within their home and educational environment. Other features which may be noted include anxieties, temper tantrums, aggressive behavior, sleep problems, and autism.
Based on findings in our cohort, individuals with SETBP1 LoF variants do not show major congenital or growth anomalies. The most frequently reported feature was young-onset vision impairment, observed in almost half of the cases, with hypermetropia being the most common problem. Ophthalmologic check-up at diagnosis should therefore be advised. Ankyloglossia and genital anomalies, such as undescended testicles, were noted in 22% and 17% of the cases. Eczema or sensitive skin was noted in 22% of the individuals. However, eczema is thought to be caused by multifactorial inheritance with a strong familial genetic component [21] and we do not have access to family histories regarding this feature.
In general, we did not identify key dysmorphisms leading to a recognizable facial gestalt that is consistent across all affected individuals. However, several individuals showed subtle overlapping facial dysmorphisms including ptosis, blepharophimosis, hypertelorism and a full nasal tip (Fig. 3). Due to the lack of very specific dysmorphic features, clinicians will often diagnose patients with SETBP1 haploinsufficiency disorder through exome sequencing or gene panels.
By applying a systematic approach to a substantive number of independent patients, this study has revealed that the phenotype of SETBP1 haploinsufficiency disorder is associated with a wider clinical severity spectrum than previously reported [14]. Seven individuals had an IQ in the normal or borderline range (IQ > 70). We could not find evidence for a specific genotype–phenotype correlation as variants of both mildly affected individuals as well as severely affected individuals were scattered throughout the gene and we did not see variant clusters of similarly affected individuals. In addition, all variants undergo NMD to the same extent leading to haploinsufficiency.
The observation of individuals at both mild and severe ends of the phenotypic spectrum, may impact the choice for genetic tests and posttest genetic counseling. A possible explanation for this variability in phenotype could also be a second hit diagnosis in some severely affected individuals. However, the majority of individuals was tested using an exome gene panel approach (Supplementary Table 1). In addition, we have tried to limit this opportunity by excluding individuals with a second molecular diagnosis. The contribution of other DNA variants in the genetic background, modulating effects related to the expression levels of the remaining wild-type allele, and nongenetic environmental exposures are other possible factors influencing the phenotypic outcome.
In conclusion, this study provided an overview of the broad clinical spectrum associated with SETBP1 haploinsuffiency and may guide counseling and management after diagnosis. Currently, the signs and symptoms of this disorder are referred to as mental retardation autosomal dominant 29 (MRD29) #616078 or SETBP1 disorder . We would suggest to use SETBP1 haploinsufficiency disorder to designate the phenotype associated with SETBP1 LoF variants. This terminology will prevent future misperceptions related to gain of function variants causing Schinzel-Giedion syndrome and better reflect the fact that not all individuals will develop intellectual disability.
Finally, further studies will need to address the pathogenicity and the underlying mechanism (gain vs loss of function) and associated clinical features of missense variants in SETBP1. Currently, this group of patients does not always know whether the clinical manifestations are due to either SGS or SETBP1 haploinsufficiency disorder.
References
Hoischen A, van Bon BW, Gilissen C, Arts P, van Lier B, Steehouwer M, et al. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat Genet. 2010;42:483–5.
Tinkle Brad TB, Christianson CA, Schorry EK, Webb T, Hopkin RJ. Long-term survival in a patient with del(18)(q12.2q21.1). Am J Med Genet Part A. 2003;119A:66–70.
Kotzot Dieter D, Kotzot D, Haberlandt E, Fauth C, Baumgartner S, Scholl-Bürgi S, et al. Del(18)(q12.2q21.1) caused by a paternal sister chromatid rearrangement in a developmentally delayed girl. Am J Med Genet Part A. 2005;135:304–7.
Feenstra I, Vissers LE, Orsel M, van Kessel AG, Brunner HG, Veltman JA, et al. Genotype-phenotype mapping of chromosome 18q deletions by high-resolution array CGH: an update of the phenotypic map. Am J Med Genet Part A. 2007;143A:1858–67.
Bouquillon S, Andrieux J, Landais E, Duban-Bedu B, Boidein F, Lenne B, et al. A 5.3Mb deletion in chromosome 18q12.3 as the smallest region of overlap in two patients with expressive speech delay. Eur J Med Genet. 2011;54:194–7.
Buysse K, Menten B, Oostra A, Tavernier S, Mortier GR, Speleman F. Delineation of a critical region on chromosome 18 for the del(18)(q12.2q21.1) syndrome. Am J Med Genet Part A. 2008;146A:1330–4.
Cody JD, Sebold C, Malik A, Heard P, Carter E, Crandall A, et al. Recurrent interstitial deletions of proximal 18q: a new syndrome involving expressive speech delay. Am J Med Genet Part A. 2007;143A:1181–90.
Filges I, Shimojima K, Okamoto N, Röthlisberger B, Weber P, Huber AR, et al. Reduced expression by SETBP1 haploinsufficiency causes developmental and expressive language delay indicating a phenotype distinct from Schinzel-Giedion syndrome. J Med Genet. 2011;48:117–22.
Marseglia G, Scordo MR, Pescucci C, Nannetti G, Biagini E, Scandurra V, et al. 372 kb microdeletion in 18q12.3 causing SETBP1 haploinsufficiency associated with mild mental retardation and expressive speech impairment. Eur J Med Genet. 2012;55:216–21.
Eising E, Carrion-Castillo A, Vino A, Strand EA, Jakielski KJ, Scerri TS, et al. A set of regulatory genes co-expressed in embryonic human brain is implicated in disrupted speech development. Mol Psychiatry. 2019;24:1065–78.
Hildebrand MS, Jackson VE, Scerri TS, Van Reyk O, Coleman M, Braden RO, et al. Severe childhood speech disorder: gene discovery highlights transcriptional dysregulation. Neurology. 2020;94:e2148–67.
Hamdan FF, Srour M, Capo-Chichi JM, Daoud H, Nassif C, Patry L, et al. De novo mutations in moderate or severe intellectual disability. PLoS Genet. 2014;10:e1004772.
Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–82.
Coe BP, Witherspoon K, Rosenfeld JA, van Bon BW, Vulto-van Silfhout AT, Bosco P, et al. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nat Genet. 2014;46:1063–71.
Fokkema IFAC, Taschner PEM, Schaafsma GCP, Celli J, Laros JFJ, den Dunnen JT. LOVD v.2.0: the next generation in gene variant databases. Hum Mutat. 2011;32:557–63.
Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat. 2015;36:928–30.
WHO. Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatr Suppl. 2006;450:86–95.
Piazza R, Magistroni V, Redaelli S, Mauri M, Massimino L, Sessa A, et al. SETBP1 induces transcription of a network of development genes by acting as an epigenetic hub. Nat Commun. 2018;9:2192.
Coban-Akdemir Z, White JJ, Song X, Jhangiani SN, Fatih JM, Gambin T, et al. Identifying Genes Whose Mutant Transcripts Cause Dominant Disease Traits by Potential Gain-of-Function Alleles. Am J Hum Genet. 2018;103:171–87.
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91.
Bieber T. Atopic Dermatitis. N Engl J Med. 2008;358:1483–94.
Acknowledgements
The authors are grateful to all the patients and their parents who collaborated on our research and gave their consent to share the clinical data and/or photos. SEF & MMK are supported by the Max Planck Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jansen, N.A., Braden, R.O., Srivastava, S. et al. Clinical delineation of SETBP1 haploinsufficiency disorder. Eur J Hum Genet 29, 1198–1205 (2021). https://doi.org/10.1038/s41431-021-00888-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-021-00888-9
This article is cited by
-
Structural rearrangements as a recurrent pathogenic mechanism for SETBP1 haploinsufficiency
Human Genomics (2024)
-
Impaired neurogenesis and neural progenitor fate choice in a human stem cell model of SETBP1 disorder
Molecular Autism (2023)
-
Identification of a novel de novo mutation of SETBP1 and new findings of SETBP1 in tumorgenesis
Orphanet Journal of Rare Diseases (2023)
-
A new impact factor for European Journal of Human Genetics
European Journal of Human Genetics (2021)