Abstract
Predictive testing for Huntington disease (HD) in 25% at-risk individuals is testing with full knowledge, and sometimes assuming, that the parent does not want to know his status. The goal of this study was to understand: (1) the differences in the motivation between 25% and 50% at-risk individuals to be tested and (2) the consequences of “double disclosure”, including parental reactions. Test requests from 25% at-risk individuals were rare (155/1611, 10%). We compared their motivation with those of 1456 50% at-risk individuals. The principal motivation to have the test for both groups was “to know” (48% versus 58%, p = 0.049), but the desire to have children was more frequent in the 25% at-risk group (32% versus 17%, p < 0.001). Sixty percent of the 25% at-risk group went through the testing procedure: 15% (n = 14) were variant positive for HD. Testees reported four adverse reactions of their parent (22%): one committed suicide and three became depressed. This result highlights the impact of “double disclosure”, a bad result for the person themselves and the transmitting parent. It is the responsibility of the team to anticipate this outcome with the 25% at-risk individuals: children revealing the genetic status to their parent. They should help the testees and their family to find a satisfactory solution to help prevent adverse reactions. This includes ensuring that the candidate is well-infomed abour the testing options and consequences to her/himself but also to her/his parent. The at-risk parent should be offered to discuss the implications of their child’s testing.
Similar content being viewed by others
Introduction
Presymptomatic testing (PT) has been available for more than 20 years for Huntington disease (HD), a genetically determined late-onset disease. HD is an autosomal dominant neurological disorder characterized by psychiatric manifestations, cognitive impairment, and movement disorders. In most of the cases, the first symptoms appear between 30 and 50 years of age, and the disease slowly progresses over decades. The Huntingtin (HTT) gene contains a trinucleotide (CAG) repeat in the first exon. The variant consists of an abnormal expansion of the repeat beyond a threshold of 35 units and is responsible for more than 90% of HD phenotypes. At-risk individuals can choose whether to have a genetic test performed for the disease that runs in their family. Generally, 50% unaffected at-risk individuals, i.e., being linked to a first-degree relative with the disease, request being tested. In this case, it is a personal and autonomous choice [1].
In rare cases, a 25% unaffected at-risk person wants to know their genetic status, even though their 50% at-risk parent does not wish to know their own genetic status. Even though counselors usually offer to meet the parent at risk, in some instances we would assume that they do not want to know. In the case of an unfavorable result, the applicant will know that the parent is variant positive for HD. The guidelines for PT in HD, updated in 2013, stipulate that “extreme care should be exercised when testing would provide information about another person who has not requested the test” and when “an individual at 25% risk requests testing with full knowledge that his/her parent does not want to know his/her status” and “every effort should be made by the counsellors and the individuals concerned to come to a satisfactory solution of this conflict” [2]. According to the literature review, requests from 25% at-risk individuals vary from less than 1% [3] to a maximum of 17.7% [4]. The median proportion of 25% at-risk requests is 6.8% (8.1 [3], 9 [5], 5.7 [6], 9.8 [7], 6.8 [8], 6% [9]). All studies that reported cases of 25% at-risk individuals in their cohort stated that the right to know was the most important reason. After offering to the at-risk relative to be tested first, they eventually accepted the request of the 25% at-risk individual. Some at-risk parents accepted to be tested before their children (from 8 to 23% in different centers) [3,4,5,6, 8,9,10]. Although there are fewer requests from 25% at-risk than 50% at-risk individuals, it is important to know the outcome and how they handled their result.
Our goal was to: (1) compare the age, motivations, and time required before deciding to have the test performed between 25% at-risk and 50% at-risk individuals, (2) compare the outcome of 25% at-risk individuals, depending on the result of the PT (variant positive or variant negative for HD), (3) observe whether revealing the parent’s status adversely affected the parent–child relationship, and (4) understand the familial context that led them to request the test before their at-risk parent.
Methods
PT for late-onset neurological diseases has been available since 1992 at the Pitié-Salpêtrière University Hospital in Paris, France. The multidisciplinary framework includes a geneticist, a psychologist, and nurse specialized in genetics. The procedure for PT is carried out in three steps: (1) information, reflection, and decision making; (2) blood sampling, testing, and result disclosure; and (3) post-test follow-up. Relevant oral and written information are both provided, including the names of professionals, a direct phone number, and an e-mail contact. After the first contact with the neurogeneticist, at least one session with a psychologist is organized before blood sampling and disclosure of the result. This is mandatory since the result will be definitive and the person will not be able to ignore it. The team provides post-test support based on the pretest wishes of the individual. All tested subjects are offered long-term follow-up as part of the PT procedure. A verbal agreement for future contact is provided by the subject at the time of test disclosure.
During the information phase, the testees fall into two categories: either 50% at risk for HD, when the parent of the individual has been clinically or genetically diagnosed; or 25% where the at-risk parent has no clinical signs, reported by the testee at the time of the PT request. At least two counseling session were offered to the 25% at-risk individuals with the geneticist and the psychologist to anticipate the impact of the results in their life and family. As part of a more familial approach, the team offers to meet the at-risk relative in those cases in order to inform them about the consequences of letting their children being tested first. When the 25% at-risk individual refuses to inform his/her at-risk parent, the team respected the guidelines which states that “if no consensus can be reached the right of the adult child to know should have priority over the right of the parent not to know” [11].
Information concerning the age when the PT was requested, the sex, at-risk parent status, marital status, whether they had children, the time between first contact and having the PT, principal motivation for requesting the PT, the test result, and CAG expansions was available for all testees.
For the study, we contacted “25% at-risk” individuals who were variant positive for HD and selected the same number of 25% at-risk individuals who were variant negative for matched comparison, based on their age and availability. They were contacted by telephone and asked to answer the following questions. (1) How were they informed about their risk, when, and by whom? (2) Why did they want to take the test before their at-risk parent? (3) Did they inform their at-risk parent and relatives about their decision to take the test and did they disclose the result? (4) What was the parent’s reaction to the test disclosure? (5) Did the test change their relationship with their parent?
Qualitative dimensions were explored through a semi-structured interview with two variant positive and two variant negative for HD individuals. We chose them based on their willingness to meet the team after the telephone interview, and that they lived close to our center. The purpose of the interview was to improve our understanding of their experiences and feelings following the telephone interview.
We compared categories using Fisher’s exact tests and means with t-tests. Results were statistically significant if p < 0.05. Data were analyzed using SPSS Statistics (Version 24.0).
Results
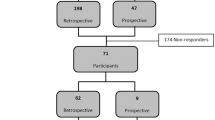
Between 1992 and 2016, 1611 individuals requested a PT for HD in our center, the Pitié-Salpêtrière University Hospital in Paris, France. They were all at risk for HD because a relative had been diagnosed with an abnormal CAG expansion of >35 in the HTT gene. There were 1456 50% at-risk and 155 (10%) 25% at-risk individuals. The parents of the 25% at-risk individuals had not been diagnosed with HD.
Sixty percent of the 25% at-risk group went through the testing procedure (94/155), which is less than the 50% at-risk individuals (73%, p < 0.001), and obtained a result: 15% (14/94) were variant positive for HD. We compared motivations of those who did take the test with those who did not. Their motivations were significantly different (p = 0.007). Interestingly, those who did not define their motivation clearly (“I don’t know”) were more frequent in the group who did not take the test (17% versus 4%) and those who desire to have children were less frequent (25 versus 40%). The wish to know was a similar frequent motivation in those who did not take the test compared to those who did (56% and 51%). We attempted to contact the variant-positive individuals after a mean of 6.5 ± 4.2 years and reached 9 of 14. Five were not reachable, three because they had an unlisted number and two because they never answered their telephone. We then contacted nine variant-negative individuals, matched for age with the variant-positive individuals.
All variant-negative individuals were married or involved in a relationship (6/9 before the test and 3/9 1 to 2 years after the result), and 7/9 had had children since the disclosure; two already had a child before being tested. In the variant-positive group, two were involved in a relationship, seven were single, and two had had children since receiving the result; three already had a child before being tested.
Comparison between 25 and 50% at-risk testees for motivation to be tested
The 25% at-risk individuals were younger than those of the 50% at-risk group (31.2 ± 9.4 years versus 35.6 ± 11.8 years, p = 0.001). The principal motivation to have the test was “desire to know” for both groups (48% versus 58%, p = 0.001), but the desire to have children was a more frequent motivation to take the test in the 25% at-risk group (32% versus 17%, p = 0.001, Table 1). Before the age of 40 years, the desire to have children was more often given as the principal motivation by the 25% at-risk group (36% versus 22%, p < 0.001). There no difference between the two groups for those who already had children (Table 2).
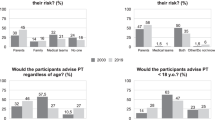
Attitudes of the family towards the 25% at-risk individuals who took the test (n=18): when and how they were informed of their risk?
The mean age when receiving the information was 23.9 ± 10.3 years. Seven of 18 testees had been informed by their transmitting parent about the risk of HD. Otherwise, the information was given by the other parent (n = 3) or another member of the family (n = 8). For two individuals, their at-risk parent had died before without symptoms of the disease. At the time of PT for HD, most (12/16) believed that their parent was not a variant-positive person before going through testing, four persons were suspecting doubtful signs, but were not affirmative.
Why did the transmitting parent not want to be tested, despite the request of the child (n=16)?
Before starting the PT process, all but one testee explained to their at-risk parent their wish to know their status for HD and the implications of being tested first, but none took the test before their child. Parental reasons, reported by the testees, were: (1) the feeling it would not change their lives (4/16), (2) conviction that they could not be variant positive (4/16), (3) fear of an unfavorable result (3/16), (4) unwillingness to know (2/16), and (5) no specific reason (3/16). When testees made their parents aware of their plans, three did not support their child.
To tell or not to tell the transmitting parent (n=16)?
When they entered PT, 9/16 25% at-risk individuals had informed their at-risk relative about this first step. After first counseling, two tried to persuade the parent to take the test first. Two parents accepted to be counseled and we did counsel them with empathy and appreciation of the reluctance to be tested. Despite our efforts, they did not want to be tested finally. Four informed their at-risk parent only after their own disclosure and one never did. All declared that they understood that their result could have an impact on their parent.
All but one testee (15/16) disclosed their result to their transmitting parents, one variant-positive individual only after 2 years. One variant-negative individual did not share the good news with his/her parent. Most (5/8) in the variant-positive group revealed their status immediately and the remaining three took 6 months, 1 year, and 2 years. All the variant-negative individuals who revealed their result did it within 6 months.
Consequences of “double disclosure”, including parental reactions for 25% at-risk individuals after testing
Testees reported four adverse reactions of their parent (22%): one committed suicide and three became depressed. The suicide occurred 1 month after the disclosure to the parent. None claimed to regret having done the PT. Testees in the variant-positive group reported that it was important to know and to not live in ignorance about their future (6/9). They felt that it was important to not recreate the same situation of their family, obliging them to take the test before their own parent.
Case reports
The first case that we report was that of a 29-year-old single individual, without children, who was the only child of a father at risk for HD. She learned about her 25% risk to be variant positive for HD when she was 12 years old. Her parents had been separated since she was 9 years old, and she lived with her mother until the age of 18 years. Her mother advised her about her risk and had always told her that it was important to know. Her ex-husband did not provide information about HD in his family until their daughter was 10 years old and she blamed him for not informing her sooner.
When she was 19 years old, she decided to take the test but waited 5 years before doing so. Her father did not take the test because he believed he would be variant negative for HD, since he did not show signs of the disease at the age of 62 years. Nevertheless, she noted that her father had tremor, but did not link this sign to HD, but rather to Parkinson disease. She had never had contact with a person with HD. Her variant-positive status came as a shock. She never believed that she could be, since her mother always told her that this was unlikely. When she revealed her status to her father, she said he just seemed surprised but not concerned. She still blamed him because of his lack of involvement and for not having taken the test before she did.
During the interview, she expressed the wish to learn more about the disease. She forgot what had been discussed during the PT and wished to meet the team again but canceled two appointments. She did not regret having been tested.
The second case was that of a 45-year-old man, living with a partner and two children, and whose father is at risk for HD. His parents separated a long time ago. He asked to be tested 1 year after he learned about his 25% risk at the age of 41 years. He informed his family about his decision to take the test instead of his father, who began but never completed the PT procedure in another center, because he assumed he would not be a variant-positive person. He was variant negative for HD and told his father immediately, but not his brother with whom he had ceased all links. His father was relieved, but he was angry with his father for not having protected him. He still believes his father should be tested, to be able to ensure his other son that he will not have HD. He expressed that he would have preferred his father to take the test before him, and always planned to disclose the result to him, regardless of whether he was variant positive for HD or not.
Discussion
In our series, 10% of testees had a 25% risk at the time they requested the test, a low number comparable to that of other studies: 7.95%, ranging from 0.7 to 17.7% [3,4,5,6, 8, 10]. In addition, only 60% asked for the result. Thus, our study had only a small number of individuals in whom to study the outcome. The 25% at-risk individuals differed from the 50% at-risk individuals, as they were younger and less often had children. They withdrew more often from the testing procedure (40 versus 27%). This may be due to their being younger and wanting to wait or postpone their decision to be tested [6, 10]. Motivations to be tested were different and showed that wanting to have children was more frequently stated by 25% at-risk individuals.
There was no difference in the CAG number in our cohort between 25 and 50% at-risk individuals, contrary to a previous report of Scuffham and MacMilla [4] in 2014, in which the frequency of CAG number of reduced penetrance (36–39 CAG repeats of the longest allele) were more often found for 25% at-risk individuals than 50% at-risk individuals (20 versus 1.2%).
The at-risk parent did not take the test, despite all but one of their children telling them of their wish to know. It may be due to the fear of an unfavorable result and having to cope with the burden of unwanted knowledge. Another interpretation is denial of the family disease; this is frequently observed in families with HD. Despite the difficulty of double disclosure, all but one, variant negative for HD, informed their parent of their result. This could be an effect of the counseling strategy, as transparency and the need to communicate are counseling themes.
Our results confirmed that “extreme care should be exercised when testing would provide information about another person who has not requested the test” as stated in the guidelines [2]. There were adverse reactions for four of the nine parents, including one suicide, related to the disclosure by their child or the disease itself [12,13,14]. The prevalence of suicide attempts in HD is from 5 to 10% [15] versus 2.7% in the general population [16]. There appear to be two critical periods of suicide attempts: first, when the individual starts to experience the disease, with mild symptoms before diagnosis, and when independence diminishes [14]. A worldwide assessment of the psychiatric reactions after PT for HD published in 1999 [13] showed that 2% of individuals who received an unfavorable result had a catastrophic reaction. None have been reported in 25% at-risk cases [5, 17], but no authors have focused on the reactions of the at-risk parent after disclosure of the result nor did they provide information on what happened. The depression in the three parents may be related either to the disease or the disclosure [18].
We did not follow the relatives of our testees, as it is likely that some effects could not have been reported to us by the 25% at-risk individuals. Moreover, we lost 5/14 individuals in the 25% at-risk group who turned out to be variant positive to follow-up. We do not know whether disclosure resulted in catastrophic events for them or their family nor whether they informed their relatives of their genetic status.
The PT of 25% at-risk individuals could be harmful for relatives following unsolicited pronouncements. Efforts should be made to encourage testees to inform their families about their wanting to know their variant positive for HD status and to involve the parent in pretest counseling. This would prepare them for unfavorable or uncertain results about their status and sometimes persuade them to take the test earlier or protect their right not to know.
The two case reports show that it is difficult to imagine the disease without knowing a person with HD, a situation that is amplified in 25% at-risk individuals. The absence of empathy of the parent intensified the feeling of betrayal, but also the guilt of revealing the status of the father. It is difficult to cope with parental renouncement when children feel obliged to reverse roles and place themselves in a parental position. The fathers were unwilling to have the test because they were in denial of the disease or afraid to cope with the result. Normally, parents inform their children of the risk and then they decide whether they want to know or not. Thus, having a parent at risk who does not want to know for themselves, but relying on the child to seek information, may express a problem in parent–child communication unrelated to the parent’s health status. This situation of the child taking the test instead of the parent, with the responsibility of revealing his status, is more related to the specific familial context. Individuals with a 25% risk require particularly careful and multistage counseling and the at-risk parent should be offered an appointment to discuss the implications of their child’s testing with the view to offer to be tested first.
References
Clément S, Gargiulo M, Feingold J, Durr A. Guidelines for presymptomatic testing for Huntington’s disease: past, present and future in France. Rev Neurol (Paris). 2015;171:572–80.
MacLeod R, Tibben A, Frontali M, Evers-Kiebooms G, Jones A, Martinez-Descales A et al. Recommendations for the predictive genetic test in Huntington’s disease. Clin Genet. 2013;83:221–31.
Dufrasne S, Roy M, Galvez M, Rosenblatt DS. Experience over fifteen years with a protocol for predictive testing for Huntington disease. Mol Genet Metab. 2011;102:494–504.
Scuffham TM, MacMillan JC. Huntington disease: who seeks presymptomatic genetic testing, why and what are the outcomes? J Genet Couns. 2014;23:754–61.
Maat-Kievit A, Vegter-Van Der Vlis M, Zoeteweij M, Losekoot M, van Haeringen A, Roos RA. Predictive testing of 25 percent at-risk individuals for Huntington disease (1987-1997). Am J Med Genet. 1999;88:662–8.
Benjamin CM, Lashwood A. United Kingdom experience with presymptomatic testing of individuals at 25% risk for Huntington’s disease. Clin Genet. 2000;58:41–49.
Creighton S, Almqvist EW, MacGregor D, Fernandez B, Hogg H, Beis J, et al. Predictive, pre-natal and diagnostic genetic testing for Huntington’s disease: the experience in Canada from 1987 to 2000. Clin Genet. 2003;63:462–75.
Baig SS, Strong M, Rosser E, Taverner NV, Glew R, Miedzybrodzka Z, et al. 22 Years of predictive testing for Huntington’s disease: the experience of the UK Huntington’s Prediction Consortium. Eur J Hum Genet. 2016;24:1515.
Tassicker R, Marshall P, Liebeck T, Keville M, Singaram B, Richards F. Predictive and pre-natal testing for Huntington Disease in Australia: results and challenges encountered during a 10-year period (1994-2003). Clin Genet. 2006;70:480–9.
Trembath MK, Tassicker RJ, Collins VR, Mansie S, Sheffield LJ, Delatycki MB. Fifteen years of experience in predictive testing for Huntington disease at a single testing center in Victoria, Australia. Genet Med J Am Coll Med Genet. 2006;8:673–80.
International Huntington Association and the World Federation of Neurology Research Group on Huntington’s Chorea. Guidelines for the molecular genetics predictive test in Huntington’s disease. J Med Genet. 1994;31:555–9.
Lawson K, Wiggins S, Green T, Adam S, Bloch M, Hayden MR. Adverse psychological events occurring in the first year after predictive testing for Huntington’s disease. The Canadian Collaborative Study Predictive Testing. J Med Genet. 1996;33:856–62.
Almqvist EW, Bloch M, Brinkman R, Craufurd D, Hayden MR. A worldwide assessment of the frequency of suicide, suicide attempts, or psychiatric hospitalization after predictive testing for Huntington disease. Am J Hum Genet. 1999;64:1293–304.
Paulsen JS, Hoth KF, Nehl C, Stierman L. Critical periods of suicide risk in Huntington’s disease. Am J Psychiatry. 2005;162:725–31.
Fiedorowicz JG, Mills JA, Ruggle A, Langbehn D, Paulsen JS, PREDICT-HD Investigators of the Huntington Study Group. Suicidal behavior in prodromal Huntington disease. Neurodegener Dis. 2011;8:483–90.
Nock MK, Borges G, Bromet EJ, Alonso J, Angermeyer M, Beautrais A, et al. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. Br J Psychiatry. 2008;192:98–105.
Lindblad AN. To test or not to test: an ethical conflict with presymptomatic testing of individuals at 25% risk for Huntington’s disorder. Clin Genet. 2001;60:442–6.
Gargiulo M, Lejeune S, Tanguy ML, Lahlou-Laforêt K, Faudet A, Cohen D, et al. Long-term outcome of presymptomatic testing in Huntington disease. Eur J Hum Genet. 2009;17:165–71.
Acknowledgements
We are grateful to the testees for accepting to respond to our questions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bonnard, A., Herson, A., Gargiulo, M. et al. Reverse pre-symptomatic testing for Huntington disease: double disclosure when 25% at-risk children reveal the genetic status to their parent. Eur J Hum Genet 27, 22–27 (2019). https://doi.org/10.1038/s41431-018-0255-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-018-0255-7
This article is cited by
-
Genetic counseling and testing practices for late-onset neurodegenerative disease: a systematic review
Journal of Neurology (2022)
-
Informing about genetic risk in families with Huntington disease: comparison of attitudes across two decades
European Journal of Human Genetics (2021)
-
Recommendations for pre-symptomatic genetic testing for hereditary transthyretin amyloidosis in the era of effective therapy: a multicenter Italian consensus
Orphanet Journal of Rare Diseases (2020)
-
Presymptomatic testing of those at 25% risk of autosomal dominant neurodegenerative disease- testing team beware
European Journal of Human Genetics (2019)