Abstract
This observational study aimed to investigate whether the reported association between family history (FH) of breast cancer (BC) or ovarian cancer (OC) and OC risks in BRCA1/2 mutation carriers can be explained by mutation position on the gene. In total, 3310 female BRCA1/2 mutation carriers participating in a nationwide prospective cohort (Hereditary Breast and Ovarian Cancer in the Netherlands) were included. FH was classified according to cancer occurrence in first-degree relatives (BC only, OC only, both, neither) and mutations were classified according to their position on the gene (OC cluster region (OCCR), BC cluster region, neither). The main outcome was OC occurrence. Cox proportional-hazard models were applied to investigate the association between FH and OC risks before and after adjusting for mutation position. Of all women included, 202 were diagnosed with OC. A BC-only FH tended to be associated with lower OC risks when compared with a FH without BC/OC (HR: 0.79, 95% CI: 0.52–1.17; HR: 0.59, 95% CI: 0.33–1.07 for BRCA1 and BRCA2, respectively) while an OC-only FH tended to be associated with higher risks (HR: 1.58, 95% CI: 0.90–2.77; HR: 1.75, 95% CI: 0.70–4.37 for BRCA1 and BRCA2, respectively). After adjusting for mutation position, association between FH and OC risks was slightly smaller in magnitude (HR: 0.85, 95% CI: 0.55–1.30; HR: 0.64, 95% CI: 0.34–1.21 for BC-only FH in BRCA1 and BRCA2, respectively; HR: 1.46, 95% CI: 0.80–2.68; HR: 1.49, 95% CI: 0.44–4.02 for OC-only FH in BRCA1 and BRCA2, respectively), indicating that mutation position explains only part of the association. Considering the magnitude of the observed trend, we do not believe FH should be used to change counseling regarding OC prevention.
Similar content being viewed by others
Introduction
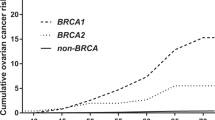
Ovarian cancer (OC) is the seventh most prevalent cancer in women worldwide [1]. Up to 24% of OC cases are due to a genetic predisposition, most often associated with BRCA1/2 mutations [2]. Numerous studies aimed at estimating the lifetime OC risk for BRCA1/2 mutation carriers [3,4,5] obtaining varying results according to the population studied and methodology applied [6], with estimates ranging between 31–59% in BRCA1 and 6–18% in BRCA2 mutation carriers [3,4,5]. Subsequent studies aimed to identify genetic and environmental factors associated with the observed risk variation in mutation carriers [7].
A family history (FH) of OC (hereafter: OCFH) has been reported to be a predictor of OC risk, with increased risks observed among relatives of OC patients in the general population (relative risks: 2.4–24.0) [8,9,10,11]. Subsequently, it was observed that also among BRCA1 mutation carriers, those with an OCFH had increased OC risks relative to those with no OCFH, whereas a FH of breast cancer (BC; hereafter BCFH) lowered OC risks [12]. This suggested that, besides the mere presence of a BRCA1/2 mutation, other factors might influence OC risk.
Shortly after BRCA1/2 were described, a possible genotype–phenotype correlation was reported [13, 14]. Mutations in central regions of BRCA1 and BRCA2 appeared to be associated with a higher incidence of OC, relative to BC incidence [15, 16]. These regions of BRCA1/2 were named “ovarian cancer cluster regions” (OCCR). The reported observations were recently confirmed in a large international study, which not only confirmed the previously described OCCRs (defining its’ boundaries more precisely), but also identified multiple breast cancer cluster regions (BCCRs). However, the study did not account for the contribution of BCFH or OCFH [17]. It remains unclear to what extent the variation in OC risks in BRCA1/2 mutation carriers is explained by mutation position, by OCFH or BCFH, or by an interaction of these variables.
In a previous study, we investigated the combined impact of BCFH or OCFH and mutation position on OC risks in women from BRCA1/2 families. OCFH was associated with increased OC risks in BRCA1 families, however, this association was no longer significant after taking mutation position into account. For women from BRCA2 families, OCFH was a predictor of OC risk independent from the mutation position [18]. The study included Dutch women from a small geographical area (which was reflected by a limited mutation spectrum) and used the initial definition of the OCCRs [15, 16], which is less precise than the recently proposed definition of OCCRs and BCCRs [17].
Because of their increased OC risk, BRCA1/2 mutation carriers are advised to have risk-reducing salpingo-oophorectomy (RRSO) before the age at which OC risk starts to rise, regardless of their FH or mutation position [19]. RRSO induces early menopause, which has several unwanted consequences [20]. Understanding the causes for variation on age-related OC risk among the group of BRCA1/2 mutation carriers who are aware of their mutation and seek counseling could enable individualized risk estimations, which could have implications for patient counseling on timing of RRSO. The present study aimed to investigate in a national Dutch cohort of female BRCA1/2 mutation carriers whether the association between cancer FH and OC risks can be explained by the mutation position on the gene.
Patients and methods
Study population
The Dutch Hereditary Breast and Ovarian Cancer study (HEBON) [21] prospectively includes subjects tested for a genetic predisposition of BC/OC with retrospective data collection and prospective follow-up. From 1999 onward, individuals undergoing genetic testing for BRCA1/2 mutations at one of the nine genetic centers in the Netherlands were invited to participate in this study. All participants were asked to fill out a questionnaire, covering information on date of birth, medical history (including personal history of OC), previous surgeries, and FH of cancer. Data regarding DNA testing were provided by the clinics and is constantly updated during follow-up, as is information on survival. The study was approved by the ethics committees of all participating centers and all patients enrolled provided written informed consent.
For the present study, we selected from the HEBON database proven female carriers of a BRCA1 or BRCA2 mutation. Participants replied to the study questionnaire between February 1999 and November 2013. Information on OC occurrence in the included patients was cross-checked with the Nationwide Network and Registry of Histo- and Cytopathology in the Netherlands (last updated in August 2014) [22] for confirmation of self-reported cases and identification of cases diagnosed after patients filled out the questionnaires. Women who had both a BRCA1 and a BRCA2 mutation (n = 14), deletions involving several domains of BRCA1/2 (n = 11), or of whom follow-up time could not be calculated because of missing information on age at OC diagnosis (n = 0), age at RRSO (n = 28), or filling out the questionnaire (n = 20) were excluded.
Data processing
Follow-up time was calculated from date of birth until date of censoring event, which could be diagnosis of OC, RRSO, or death; whichever occurred first. Women in whom no censoring events occurred were censored at date of completion of the questionnaire. Women were considered to have had RRSO when they reported surgical removal of both ovaries, regardless of whether the ovaries were removed at the same or at different time points. When ovaries were removed at different time points, women were censored at the time of second oophorectomy. The number of women-years at risk was computed for each study participant from age 18 until censoring.
Occurrence of BC and OC in the family, as reported in the questionnaire, was recorded for each first-degree female relative (FDR). Cancer FH in FDR was initially classified in four categories: no BC or OC, BC cases only, OC cases only, or both BC and OC cases. To further investigate the effect of FH on OC risks, families were subsequently classified in two other ways: families with BC cases in FDRs vs. families without BC cases in FDRs (regardless of the occurrence of OC), and families with OC cases in FDRs vs. families without OC cases in FDRs (regardless of the occurrence of BC).
Mutation position was classified based on the recent publication by Rebbeck et al. [17], which proposed a classification based on the creation of several bins of mutations according to base-pair location across BRCA1 and BRCA2. Bins with significant differences in the ratio of BC vs. OC hazard ratios (RHR) were considered to be BCCR or OCCR. In this way, in the BRCA1 gene BCCR1 (nucleotides 179–505), OCCR (nucleotides 1380–4064), BCCR2 (nucleotides 4328–4945), and BCCR2’ (nucleotides 5261–5563) were defined, as well as BCCR1 (nucleotides 0–596), BCCR1’ (nucleotides 772–1806), OCCR1 (nucleotides 3249–5681 and 5946), OCCR2 (nucleotides 6645–7471), and BCCR2 (nucleotides 7934–8904) in the BRCA2 gene. Due to the relatively small number of participants in our study as compared with the Rebbeck paper, for the sake of this analysis, we classified mutations in three categories only: OCCR, BCCR, or neither OCCR nor BCCR. When there were two BCCR regions in a segment of the gene, interrupted by a segment with nonsignificant differences in RHR, this “in between” segment was considered as part of that BCCR region. The same applied for OCCR. In this way in each BRCA gene, we have only one BCCR in the 5’ end and one in the 3’ end, and one OCCR in the center. All mutations, including frameshift mutations, were classified according to their base-pair location in the gene.
Data analysis
Characteristics of study participants, frequency of OC, types of FH, and mutation position were described separately for women with BRCA1 and BRCA2 mutations. T-tests and chi-square tests were applied to compare BRCA1 and BRCA2 mutation carriers regarding these characteristics. The outcome measure of this study was the occurrence of OC. The number of OC cases per 1000 women-years at risk was described for different age groups according to each category of FH and of mutation position.
The impact of BCFH and OCFH on the age-related penetrance of OC was assessed with univariate Cox proportional-hazard models, and so was the impact of mutation position. Subsequently, multivariable Cox proportional-hazard models were applied to investigate the association between each of the proposed classifications of FH and OC risk adjusted for the mutation position. To explore the effect of birth cohorts (classified as birth before 1940 vs. birth in 1940 or later) and of a personal history of BC (and in this way a possible protective effect of previous chemotherapy on OC risk) on the association between FH and OC risk, these factors were also added to the models. The assumption of proportionality was checked by assessment of log minus log plots. Results were expressed as hazard ratios (HRs) with 95% confidence intervals (95% CIs).
To assess the risk of bias, three sensitivity analyses were performed. Because no data imputation method was applied, in the main analyses only women with complete information on the factor(s) assessed in each model were considered in that specific model. In sensitivity analysis 1, women with incomplete information on FH or mutation position were excluded. Because patients are often referred for genetic testing because of an OC diagnosis, OC risks might be overestimated due to ascertainment/referral bias. In sensitivity analysis 2, women diagnosed with OC before testing positive for a BRCA1/2 mutation were excluded. Finally, because information regarding cancer FH was derived through questionnaires, which were filled out during a different time frame than data regarding cancer occurrence was retrieved from the national pathology database, FH at the time of cancer diagnosis could be outdated, which could lead to misclassification bias. On sensitivity analysis 3, women who filled out the study questionnaire >1 year before being diagnosed with OC were excluded. All tests performed previously were repeated in these subsets of patients. OC incidence per 1000 women-years at risk and the respective 95% CIs were estimated in R-Studio Version 0.98.1091 [23]. All other statistical analyses were performed with IBM SPSS version 20.0 (IBM Corp., NY, USA). All tests were two-sided and p-values < 0.05 were considered significant.
Results
A total of 3383 BRCA1/2 mutation carriers were identified. Fourteen women were excluded for having both a BRCA1 and BRCA2 mutation, 11 for having a large deletion and 48 for missing information on follow-up time. In total 3310 mutation carriers (2147 BRCA1 and 1163 BRCA2) were included in the analysis (Fig. 1), among whom 202 cases of OC were diagnosed (195 self-reported cases and 7 additional cases identified through the national pathology database; of self-reported cases 93.8% were confirmed by pathology). The mean age at OC diagnosis among BRCA1 mutation carriers was 52.0 years (SD: 9.4), which was not significantly different from the mean age at diagnosis in BRCA2 mutation carriers (53.5 years; SD: 10.2). Of all women included, 1 867 had undergone a RRSO at a mean age of 45.7 (SD: 8.7) in BRCA1 and 48.9 (SD: 9.1) in BRCA2 mutation carriers (Table 1).
The incidence of OC per 1000 women-years at risk was overall higher in women with only OCFH (i.e., no BCFH) than in women with only BCFH (i.e., no OCFH) in all age groups, although CIs were wide and overlapping. Regarding mutation position, in most age groups OC was more frequent in women with OCCR mutations than in women with BCCR mutations (Table 2), which is in agreement with previous findings.
Univariate analyses indicated that OC risk in BRCA1 and BRCA2 mutation carriers tended to be affected by the type of FH. A FH with BC cases only tended to be associated with lower OC risks when compared with a FH without BC or OC cases (HR: 0.79 95% CI: 0.52–1.17 for BRCA1 and HR: 0.59, 95% CI: 0.33–1.07 for BRCA2 mutation carriers). On the contrary, a FH with OC cases only tended to be associated with higher OC risks than a FH with no BC or OC (HR: 1.58, 95% CI: 0.90–2.77 for BRCA1 and HR: 1.75, 95% CI: 0.70–4.37 for BRCA2 mutation carriers). The univariate analyses also revealed that overall, mutation position tended to be associated with OC risks for BRCA2 (HR: 1.67, 95% CI: 0.77–3.62 for OCCR and HR: 0.45, 95% CI: 0.14–1.51 for BCCR, mutations outside OCCR/BCCR as reference, p-value: 0.03 in the univariate model), but not BRCA1 mutation carriers (HR: 1.28, 95% CI: 0.76–2.15 for OCCR and HR: 1.09, 95% CI: 0.65–1.82 for BCCR, mutations outside OCCR/BCCR as reference, p-value: 0.58 in the univariate model; Table 3).
In the multivariate analyses including FH and mutation position, the impact of a BC-only and of an OC-only FH on OC risks when compared with a FH without BC or OC presented comparable magnitude, with changes in HR ranging from 0% to 30% when compared with the univariate models (HR: 0.85, 95% CI: 0.55–1.30 and HR: 0.64, 95% CI: 0.34–1.21 for a BC-only FH in BRCA1 and BRCA2 mutation carriers, respectively; HR: 1.46, 95% CI: 0.80–2.68 and HR: 1.49, 95% CI: 0.55–4.02 for an OC-only FH in BRCA1 and BRCA2 mutation carriers, respectively; Table 3), although after taking mutation position into account neither of the models were significant. In comparison with the univariate model including mutation position only, adding information on FH to the models significantly improved model fit for BRCA1 (p < 0.001) but not for BRCA2 mutation carriers (p = 0.06). Multivariate models including information not only on FH and mutation position, but also on birth cohorts and a personal history of BC yielded comparable results, with changes in HR mostly lower than 10% (Supplementary Table 1).
Sensitivity analyses 1 (excluding women with missing information) and 3 (excluding women who replied to the questionnaire >1 year before being diagnosed with OC) yielded results comparable to the main analyses. In sensitivity analyses 2 (excluding women diagnosed with OC before testing positive for a BRCA1/2 mutation), the direction of the associations observed was overall similar to the main analyses. However, due to small numbers, results were not significant (Supplementary Tables 2, 3 and 4).
Discussion
In this multicenter study including 3310 women with a BRCA1 or BRCA2 mutation, we observed that, when compared with FH without BC or OC cases, a BC-only FH among FDRs tended to be associated with lower OC risks (HR: 0.79, 95% CI: 0.52–1.17 for BRCA1 and HR: 0.59, 95% CI: 0.33–1.07 for BRCA2), whereas a FH with OC cases only tended to be associated with higher OC risks (HR: 1.58, 95% CI: 0.90–2.77 for BRCA1 and HR: 1.75, 95% CI: 0.70–4.37 for BRCA2). After taking information on mutation position into account, the association between FH and OC risks presented comparable magnitude (HR: 0.85, 95% CI: 0.55–1.30 and HR: 0.64, 95% CI: 0.34–1.21 for a FH of BC only among BRCA1 and BRCA2 mutation carriers, respectively; HR: 1.46, 95% CI: 0.80–2.68 and HR: 1.49, 95% CI: 0.55–4.02 for a FH of OC only among BRCA1 and BRCA2 mutation carriers, respectively). This indicates that the position of the mutation in the gene in three categories may explain part, but not all of the FH impact on OC risks.
Our results are in line with a recent study reporting a nonsignificant trend toward an increased risk of OC among BRCA1/2 mutation carriers with a FH of OC compared with those without a FH of the disease (HR: 1.24, 95% CI: 0.75–2.03 for BRCA1 and HR: 1.26 95% CI: 0.43–3.69 for BRCA2 women with one affected relative) [24]. Furthermore, the magnitude of the impact of a BCFH on OC risks in BRCA1/2 mutation carriers observed in the present analyses is comparable to a study by Metcalfe et al. They reported a HR of 0.75 per affected first- or second-degree relative (SDR) with BC, compared with no BCFH, among BRCA1 mutation carriers, although the number of cases in BRCA2 women was too low for analysis [12]. Our study does not consider the number of affected relatives, however, in the present study only 25% of women with BCFH and 6% of women with OCFH reported >1 relative with the disease.
The results observed in this study partly contradict our previous observation regarding the impact of type of cancer FH on OC risks adjusted for mutation position [18]. We previously reported that a FH with OC only (no BC) was associated with increased OC risks when compared with families with BC cases (regardless of the occurrence of OC) independently from mutation position on the gene in BRCA2 (HR: 4.48, 95% CI: 2.28–8.81), but not in BRCA1 mutation carriers [18]. This discrepancy may be due to population and methodological differences between the two studies. The previous study was smaller, contained on average larger families, the information on FH of cancer was acquired through the genetics file and included data on FDRs and SDRs. For the present study, information on FH was acquired for FDRs only (information for SDRs was not available), through a self-administered questionnaire, whereas self-reported information on the occurrence of OC in the included patients was validated. Although self-administered questionnaires may be associated with lower accuracy and completeness of data, information for FDRs tends to be more accurate than for SDRs [25]. Furthermore, the discrepancy between the present and the previous study may be due to differences in mutation spectrum. A list with all the mutations identified in the current study and the number of women with each specific mutation is provided on supplementary file 1.
The magnitude of the positive correlation between mutation position (OCCR, BCCR, or neither) and OC risk observed in the present study (HR:1.28 for BRCA1-OCCR mutations and HR:1.67 for BRCA2-OCCR mutations, compared with mutations outside OCCR/BCCR) is in line with a previous report using retrospective data [17]. Although a genotype–phenotype correlation in BRCA1/2 mutation carriers has been first reported >20 years ago [13, 14], it is based solely on epidemiological observations. The biological mechanisms behind the epidemiological observations remain speculative.
The results of this study indicate that mutation position explains part, but not all of the association between FH and OC risk. Other genetic and environmental risk modifiers in BRCA1/2 mutation carriers have been suggested [26]. Special attention has been recently given to genetic modifiers identified either through candidate gene studies or genome-wide association studies. Common variants related to OC risk in the general population have been also associated with OC risk in BRCA1/2 mutation carriers [27]. Moreover, loci that modify OC risk specifically in BRCA1/2 mutation carriers were also described [7, 28]. Several genes may contribute to OC risk in a polygenic manner, and it has been suggested that polygenic risk scores could be incorporated into risk prediction models in order to provide more personalized OC risk estimations for BRCA1/2 mutation carriers [29]. Furthermore, there might be other yet unknown genes associated with higher risks of OC.
One limitation of this study is that it included only women referred to family cancer clinics, and therefore might not be representative of the entire population of BRCA1/2 mutation carriers. However, the aim of this study was to provide information regarding OC risk variation specifically for this very population that is counseled in family cancer clinics. Being a highly selected group, they may differ from population based BRCA1/2 mutation carriers. A second limitation is that the number of OC cases was relatively small, especially on sensitivity analyses 2, likely due to the relatively young mean age of this cohort (47.8 years), to the proportion of women who had undergone RRSO (56.4%), a procedure associated with up to 96% reduction of OC risks, or to other competing causes of death [30]. The small number of OC cases may have limited the power of the statistical analysis. When interpreting the results it is also important to consider that several models were performed and no statistical method was applied to account for multiple testing. Furthermore, a considerable proportion of women (32.4%) reported no OCFH or BCFH. This may be due to limiting the FH information to FDRs only: it is possible that these women have SDRs with BC and/or OC. The families of the study participants were relatively small (median of two female FDRs), and may not be as informative as larger families. We did not investigate the possible effect of family clustering in our results, however, we do not expect this to have a major impact on the observed associations, as the number of carriers per family was small [6, 18].
Strengths of this study are the large number of mutation carriers included, and the high reliability of information on cancer incidence and clinical characteristics of the included women. Another strength is the use of sensitivity analyses to evaluate possible bias on our results. Results of sensitivity analyses were generally in line with those observed in the main analyses, indicating that the effect of possible bias, if present, is likely to be small.
In conclusion, a FH of BC tended to be associated with a lower OC risk while a FH of OC tended to be associated with higher OC risks in BRCA1/2 mutation carriers. This observed trend was only partly explained by the mutation position effect. Therefore, more research to identify (epi)genetic factors explaining the variation in OC risk is needed, before a more individualized counseling can be offered. Currently, RRSO is the only effective strategy to reduce OC risk, which is generally offered to women with an OC risk of >10%. Tailored risk estimation is paramount for adequate counseling on the indication and timing of this procedure. Although, considering the magnitude of the trends observed in this study, we do not believe that specific types of FH would be associated with lifetime risks low enough to change the recommendation of RRSO for BRCA1/2 mutation carriers, our observations could be taken into account when discussing the timing of RRSO with women considering to go through this procedure. Furthermore, the results from this study may be relevant for the improvement of existing algorithms used for cancer risk estimation in BRCA1/2 mutation carriers [31, 32].
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108:18032–7.
Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–33.
Mavaddat N, Peock S, Frost D, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105:812–22.
Brohet RM, Velthuizen ME, Hogervorst FB, et al. Breast and ovarian cancer risks in a large series of clinically ascertained families with a high proportion of BRCA1 and BRCA2 Dutch founder mutations. J Med Genet. 2014;51:98–107.
Vos JR, Hsu L, Brohet RM, et al. Bias correction methods explain much of the variation seen in breast cancer risks of BRCA1/2 mutation carriers. J Clin Oncol. 2015;33:2553–62.
Milne RL, Antoniou AC. Modifiers of breast and ovarian cancer risks for BRCA1 and BRCA2 mutation carriers. Endocr Relat Cancer. 2016;23:T69–84.
Soegaard M, Frederiksen K, Jensen A, et al. Risk of ovarian cancer in women with first-degree relatives with cancer. Acta Obstet Gynecol Scand. 2009;88:449–56.
Hemminki K, Granstrom C. Familial invasive and borderline ovarian tumors by proband status, age and histology. Int J Cancer. 2003;105:701–5.
Lee JS, John EM, McGuire V, et al. Breast and ovarian cancer in relatives of cancer patients, with and without BRCA mutations. Cancer Epidemiol Biomark Prev. 2006;15:359–63.
Stratton JF, Pharoah P, Smith SK, Easton D, Ponder BA. A systematic review and meta-analysis of family history and risk of ovarian cancer. Br J Obstet Gynaecol. 1998;105:493–9.
Metcalfe K, Lubinski J, Lynch HT, et al. Family history of cancer and cancer risks in women with BRCA1 or BRCA2 mutations. J Natl Cancer Inst. 2010;102:1874–8.
Gayther SA, Warren W, Mazoyer S, et al. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995;11:428–33.
Gayther SA, Mangion J, Russell P, et al. Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet. 1997;15:103–5.
Thompson D, Easton D, Breast Cancer Linkage Consortium. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68:410–9.
Thompson D, Easton D, Breast Cancer Linkage Consortium. Variation in BRCA1 cancer risks by mutation position. Cancer Epidemiol Biomark Prev. 2002;11:329–36.
Rebbeck TR, Mitra N, Wan F, et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA. 2015;313:1347–61.
Teixeira N, Mourits MJ, Vos JR, et al. Ovarian cancer in BRCA1/2 mutation carriers: the impact of mutation position and family history on the cancer risk. Maturitas. 2015;82:197–202.
National Comprehensive Cancer Network (NCCN): NCCN clinical practice in oncology (NCCN guidelines). Genetic/familial high-risk assessment: breast and ovarian. Version 2.2017. 2016; 2017.
Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483–91.
Pijpe A, Manders P, Brohet RM, et al. Physical activity and the risk of breast cancer in BRCA1/2 mutation carriers. Breast Cancer Res Treat. 2010;120:235–44.
Casparie M1, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29(1):19-24
RStudio: Integrated development environment for R (Version 0.98.1091) [Computer software]. Boston, MA. Retrieved December 2014.
Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–16.
Tehranifar P, Wu HC, Shriver T, Cloud AJ, Terry MB. Validation of family cancer history data in high-risk families: the influence of cancer site, ethnicity, kinship degree, and multiple family reporters. Am J Epidemiol. 2015;181:204–12.
Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
Kuchenbaecker KB, Ramus SJ, Tyrer J, et al. Identification of six new susceptibility loci for invasive epithelial ovarian cancer. Nat Genet. 2015;47:164–71.
Couch FJ, Wang X, McGuffog L, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet. 2013;9:e1003212.
Kuchenbaecker KB, McGuffog L, Barrowdale D et al. Evaluation of polygenic risk scores for breast and ovarian cancer risk prediction in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2017; 109: https://doi.org/10.1093/jnci/djw302.
Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–87.
Antoniou AC, Pharoah PP, Smith P, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91:1580–90.
Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145–58.
Author contributions
Study concept: all authors. Study design: GHdB, MJEM, JRV, and JCO. Data collection: HEBON. Data analysis and interpretation: NT, GHdB, MJEM, JCO, and AvdH. Manuscript preparation: NT, GHdB, MJEM, and JCO. Manuscript review: all authors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Teixeira, N., van der Hout, A., Oosterwijk, J.C. et al. The association between cancer family history and ovarian cancer risk in BRCA1/2 mutation carriers: can it be explained by the mutation position?. Eur J Hum Genet 26, 848–857 (2018). https://doi.org/10.1038/s41431-018-0111-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-018-0111-9