Abstract
Background
The Palaeolithic diet (PD) has gained popularity globally. There is emerging evidence of its putative health benefits as short-term effects on chronic diseases have been reported. We evaluated the association between long-term adherence to the PD and breast cancer (BC) risk among postmenopausal women.
Methods
65,574 women from the Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale (E3N) cohort were followed from 1993 to 2014. Incident BC cases were identified and validated. The PD score was calculated using dietary intake self-reported at baseline (1993) and follow-up (2005) or baseline only if censored before follow-up. Multivariable Cox proportional hazards regression models were used to estimate BC hazard ratios (HR) and 95% confidence intervals (CI).
Results
Over a mean follow-up of 20 years, 3968 incident BC cases occurred. An increase of 1 standard deviation in the PD score was associated with an 8% lower BC risk, fully-adjusted model: HR1-SD 0.92, 95% CI; 0.89, 0.95. Compared to women with low adherence to the PD, women with high adherence had a 17% lower BC risk, HRQ5 vs Q1 0.83, 95% CI; 0.75, 0.92, Ptrend < 0.01. When considering BC subtypes, we observed the same pattern of association (Pheterogeneity > 0.10 for all).
Conclusions
High adherence to a PD characterised by fruit, vegetables, nuts, fish, and lean meat and limited in dairy, grains, legumes, refined sugar, and alcohol was associated with a lower BC risk. The lack of heterogeneity according to BC subtypes could indicate the involvement of non-hormonal mechanisms. The protocol is registered at clinicaltrials.gov as NCT03285230.
Registry
The protocol is registered at clinicaltrials.gov as NCT03285230.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer and one of the leading causes of cancer death in women [1, 2]. According to the Global Cancer Observatory, BC accounted for 11.6% of all female cancers [3], yielding a considerable burden on healthcare and loss of Disability Adjusted Life Years [4, 5]. Much of the burden of cancer is traced to modifiable risk factors [6]; hence, identifying factors associated with BC is valuable for devising strategies for primary prevention.
One of the main modifiable lifestyle factors in relation to BC risk is diet. The Palaeolithic diet (PD), based on the notion of food components consumed during the Palaeolithic period, such as lean meat, fish, fruit, vegetables, and nuts, excluding grains, dairy, and processed foods, has gained rapid popularity in the last few years. Beneficial influences of the PD have been reported in relation to cardiovascular risk factors [7], inflammatory disease [8, 9], and colorectal tumours [10].
Studies have shown that individual dietary components such as fat [11], fruit and vegetables [12], folate [13], and dietary patterns such as Western diet [14, 15], prudent diet [14], traditional diet patterns [16], and modified Nordic Dietary Index [17] may have differential associations with BC risk overall and/or according to subtypes. However, studies on long-term adherence to the PD and BC risk are scarce; the only prospective study reported no association [18].
Therefore, we investigated the associations between the PD and incident BC among postmenopausal women, considering potential associations by oestrogen (ER) and progesterone (PR) receptor status, and cancer histology, within the Etude Epidémiologique auprès de Femmes de la Mutuelle Générale de l’Education Nationale (E3N) cohort.
Materials and methods
The E3N cohort, initiated in 1990, involves 98,995 French women aged 40 to 65 years at inclusion and selected from the health insurance scheme, which covers workers in the National Education System and their families [19]. The study participants provided written informed consent, and the cohort study received ethical approval from the French National Commission for Computerized Data and Individual Freedom. Participants were enroled in the cohort through a self-administered questionnaire followed by questionnaires every 2 to 3 years.
In the present study, follow-up began on the return date of the first food frequency questionnaire (FFQ) for women who were already menopausal at that time or the date of menopause if it occurred later. Premenopausal women free of BC contributed to the Cox model person-time at the time they attained menopause. Women contributed person-time until the date of diagnosis of any type of cancer except basal cell carcinoma, the date of the last completed questionnaire, or the date on which the last available follow-up questionnaire was mailed (November 17, 2014) whichever occurred first [20].
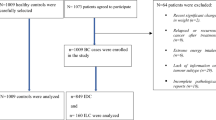
Among 74,522 women who returned the FFQ sent in 1993, we first excluded women with undefined menopausal status (n = 14), those who had never menstruated (n = 6), prevalent cancer cases (n = 4709), women with incomplete or absent follow-up information (n = 623); we further excluded women with extreme energy intake values (i.e., the 1st and 99th percentiles of the energy intake over energy requirement distribution in the population) (n = 1364), those with missing BC receptor status (n = 1309), and those who had not attained menopause at the end of follow-up (n = 923). Hence, our final study population included 65,574 women (Supplementary Fig. 1).
Dietary assessment
Dietary data were collected at the third (1993), and eighth (2005) validated self-administered FFQs [21, 22]. Participants were asked about the frequency of consumption for eight eating moments from breakfast to after-dinner snacks over the preceding year. Portion sizes were assessed via photographs and qualitative questions on specific food and drink items according to French meal patterns. Nutrient and energy intakes were obtained using the Food Composition Database derived from the French Information Centre on Food Quality [23].
The Palaeolithic diet score
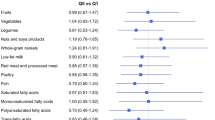
The PD score reflected the adherence to foods that emulated the evolutionary dietary pattern of the Palaeolithic era [10]. Briefly, food items were classified as characteristic of the PD (vegetables, fruit, fruit and vegetable diversity score, lean meat, fish, nuts, and calcium) or less characteristic of the PD (red [fatty] and processed meat, dairy foods, sugar-sweetened beverages, baked goods, grains and starches, sodium, and alcohol) (Supplementary Table 1). The fruit and vegetable diversity score, a proxy indicator of nutrient adequacy [24], corresponded to the participant’s consumption of the number of components of the fruit and vegetable group. Calcium intake independent of dairy foods was estimated by the residual method. The final PD score ranged from 14 to 70 (lowest to highest adherence). Recently, this score has been developed in the E3N cohort using baseline dietary data [25]; however, in the present study, the average of dietary pattern scores at baseline and follow-up were used for participants with repeated measures of diet. Baseline scores were used if participants were censored before the follow-up FFQ. For the analysis, the baseline and cumulative average scores were used for 12,689 and 52,885 participants, respectively.
Incident breast cancer ascertainment
All potential cases of BC self-reported through baseline and follow-up questionnaires (3rd to 11th) were systematically investigated. A few cases were further identified from the insurance database files and death certificates. Tumour characteristics were confirmed using original clinical and pathology reports. We included the cases for which pathology reports were unobtainable in our analysis as the proportion of false-positive self-reports was very low (<5%).
Covariates
Education, physical activity, and smoking status were self-reported at baseline. The use of oral contraception and menopausal hormone therapy (MHT) was assessed from baseline and follow-up questionnaires. Parity and family history of BC were self-reported. Body mass index (BMI) was assigned according to the value reported at baseline; self-reported height and weight were used to calculate BMI (kg/m2). In the cohort, self-reported anthropometry is considered reliable from a validation study [26]. Alcohol consumption and energy intake were calculated from the E3N FFQs.
Statistical analysis
Baseline characteristics overall and by PD quintiles were described using means and standard deviations (SD) for continuous variables and frequencies for categorical variables.
Hazard ratios (HR) and 95% confidence intervals (CI) of the BC risk were estimated using Cox proportional hazards regression models with age as the time scale (entry time: age at menopause). The following variables selected a priori were considered as potential confounders: education (≤ undergraduate, graduate, ≥ postgraduate), smoking (current, former, non-smoker), family history of BC (yes, no), physical activity (continuous), age at menarche (continuous), age at first full-term pregnancy (nulliparous, <30 years, ≥ 30 years), breastfeeding (yes, no, unknown), past history of benign breast disease (yes, no), ever use of oral contraception (yes, no), ever use of MHT (yes, no), mammography in the previous follow-up cycle (yes, no), total energy intake (excluding alcohol, continuous kcal/day), and BMI (continuous). Birth cohorts were composed of 5-year categories (<1930, 1930–1934, 1935–1939, 1940–1944, ≥1945).
The dependence of BC onset on the PD score was modelled in three ways. First, we reported HR for a 1-SD increase in the score. Second, restricted cubic splines were fitted to the fully adjusted model (five knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles of the PD score) to test for non-departure from the linear association [27]. Lastly, the PD score was categorised into quintiles; the first quintile served as the reference group. All models were stratified by 5-year birth cohorts.
Four Cox models were built: Model 1 was adjusted for age. Model 2 additionally included physical activity, education, smoking status, and family history of BC. Model 3 additionally included age at menarche, age at first childbirth, breastfeeding, ever use of MHT, ever use of contraceptive pills, past history of benign breast disease, and mammography in the last follow-up cycle, and Model 4 additionally included potential mediators BMI and energy intake. The P-value for the linear trend was estimated using the median score in each quintile. Subtypes of BC were studied in separate Cox models. We used the Q statistic to test the homogeneity of the results between the subtypes [28].
In addition, we tested for effect modification by BMI and MHT using models that included an interaction term for the variable of interest and the PD score separately and by stratification. Missing observations were < 5% for all variables except for ever breastfed and therefore were imputed to the median (for continuous variables) or modal value (for categorical variables). For ever breastfed, a ‘missing’ category was created to maintain the same number of participants in the analyses. All tests of statistical significance were two-sided, with statistical significance set at P < 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Sensitivity analyses
First, covariates with missing values were imputed with multiple imputation (Markov chain Monte Carlo Method). Second, the participants with BC diagnosed in the first five years of follow-up were excluded to overcome the effects of reverse causation. Third, since alcohol is a well-established risk factor for BC [29], we analysed the PD score without alcohol and inserted it as a covariate. Fourth, we used two additional approaches for analysing dietary measurements: baseline diet only and a time-dependent approach across the follow-up. Lastly, as Cordain et al. showed that contemporary PDs are deficient in calcium, we analysed the PD score with the exclusion of calcium [30]; furthermore, we included eggs as characteristic of the PD as proposed by Frassetto et al. to assess the influence on BC risk [31].
Results
Over a mean follow-up of 20 years, 3968 incident BC cases that could be classified by receptor and histological subtypes were diagnosed among 65,574 women. The characteristics of the women overall and according to quintiles of the PD are described in Table 1. Participants’ mean age was 52.8 (6.6) years. Compared with women in the lowest quintile of the PD, women in the highest quintile were older, more likely to be non-smokers and physically active, had marginally lower BMI, and were more likely to have breastfed, a history of benign breast disease, mammography in the last follow-up cycle, and history of menopausal hormonal therapy use. However, they had lower energy intake and were less likely to be nulliparous and have a BC family history.
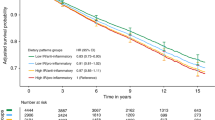
The fully adjusted model showed that the PD score was associated with lower BC risk (HR1-SD 0.92, 95% CI; 0.89, 0.95) (Table 2 and Fig. 1). Spline analyses confirmed no departure from a linear association (Pnonlinear = 0.18) (Fig. 2). When considering quintiles, we found that the PD score was inversely and linearly associated with BC risk (HRQ5 vs Q1 0.83, 95% CI; 0.75, 0.92, Ptrend < 0.01) (Table 2).
Hazard ratios (Model 4)1 for a 1-standard-deviation increase in score was presented in the figure. HR hazard ratio, CI confidence interval, ER oestrogen receptor, PR progesterone receptor. 1HR adjusted for age (as the time-scale), educational level, physical activity, smoking status, family history of breast cancer, breastfeeding, age at menarche, age at first full-term birth, past history of benign breast disease, ever use of the contraceptive pill, ever use of menopausal hormone therapy, mammography in last follow-up cycle, body mass index, and energy intake (model stratified by birth cohort).
Risk estimates were adjusted for age (as the time-scale), educational level, physical activity, smoking status, family history of breast cancer, breastfeeding, age at menarche, age at first full-term birth, past history of benign breast disease, ever use of the contraceptive pill, ever use of menopausal hormone therapy, mammography in last follow-up cycle, body mass index, and energy intake (model stratified by birth cohort). The solid blue line represents the hazard ratio, and the dashed lines the lower and upper 95% confidence interval.
When assessing the association of the PD by BC subtypes, we did not observe heterogeneity by either receptor status or histology (Pheterogeneity > 0.10 for all). For example, when we compared the risks associated with 1-SD of the PD for ER-positive and ER-negative tumours, we observed 9% and 4% lower risks, respectively (Model 4: HR1-SD 0.91, 95% CI; 0.88, 0.95 and HR1-SD 0.96, 95% CI; 0.89, 1.04) (Supplementary Table 2 and Fig. 1). As quintiles, the HRQ5 vs Q1 (95% CI) were 0.82 (0.73, 0.91, Ptrend < 0.01) and 0.90 (0.71, 1.14, Ptrend = 0.47), respectively.
The PD score was associated with both PR-positive (HR1-SD 0.92, 95% CI; 0.88, 0.96) and PR-negative tumours (HR1-SD 0.92, 95% CI; 0.87, 0.97) (Supplementary Table 2 and Fig. 1); as quintiles, the HRQ5 vs Q1 (95% CI) were 0.84 (0.74, 0.95), Ptrend < 0.01 and 0.82 (0.69, 0.97), Ptrend < 0.01, respectively. For histological subtypes, we found that the PD was associated with both ductal (Model 4: HR1-SD 0.93, 95% CI; 0.90, 0.97) and lobular (HR1-SD 0.88, 95% CI; 0.81, 0.95) tumours (Supplementary Table 4 and Fig. 1); as quintiles, HRQ5 vs Q1 (95% CI) were 0.85 (0.76, 0.96), Ptrend < 0.01, and 0.75 (0.59, 0.94), Ptrend < 0.01, respectively.
Overall, there was no interaction between the PD score and BMI categories (< 20, 20–24.99, and ≥ 25 kg/m²) (Pinteraction = 0.63) or ever use of MHT (Pinteraction = 0.34) (Supplementary Tables 5 and 6 and Supplementary Fig. 2).
The results remained similar when using multiple imputations to handle missing covariates and excluding participants diagnosed in the first five years of follow-up (results not tabulated). After excluding alcohol from the PD score, the results were not materially different, HR1-SD 0.96, 95% CI; 0.93, 0.99 and HRQ5 vs Q1 0.90, 95% CI; 0.81, 0.99, Ptrend < 0.01. When using the baseline score and the time-dependent analysis approach, the HRs were as follows, Model 4: HR1-SD 0.94, 95% CI; 0.91, 0.97 and HRQ5 vs Q1 (95% CI); 0.86 (0.78, 0.96), Ptrend < 0.01 (Supplementary Table 7), and HR1-SD 0.986, 95% CI; 0.978, 0.994 and HRQ5 vs Q1 (95% CI); 0.97 (0.94, 0.99) (Supplementary Table 8), respectively. Lastly, results were materially unchanged when excluding calcium, HRQ5 vs Q1 0.83, 95% CI; 0.75, 0.92, or when including eggs, HRQ5 vs Q1 0.80, 95% CI; 0.73, 0.88.
Discussion
Higher adherence to the PD was inversely associated with BC risk in this prospective cohort study of postmenopausal women followed for approximately 20 years. There was no significant departure from a linear relationship. We observed neither heterogeneity across subtypes nor an effect modification by BMI and MHT. These results suggest that PD could have a preventive effect on all types of BC, thus acting independently of hormonal mechanisms. We observed that the cumulative averages yielded stronger associations compared to the baseline diet and time-dependent approaches, which could be attributed to the fact that long-term adherence to a dietary pattern is more intuitive and reduces measurement errors.
To our knowledge, one previous study has examined the association between the PD and overall BC risk. Among 96,959 US women recruited in the California Teachers Study (CTS; 1995–2011), Haridass et al. observed that the Palaeolithic Index was not associated with BC risk [18]. This study and ours share common characteristics, such as a prospective design and a large sample size. However, there were some differences in the number of food groups and the inclusion or not of eggs, the fruit and vegetable diversity score, calcium, and sodium, and adjusting on confounders race and socioeconomic status in the CTS could have influenced results. Although heterogeneity regarding the inclusion of certain items in the PD has been observed in the literature [10, 32,33,34,35,36,37], our results are comparable to studies with a similar PD score showing inverse associations with colorectal adenoma and mortality [10, 36].
We demonstrated the protective influence of the PD in women at risk of BC. Similarly, beneficial effects of the PD have also been reported in BC patients in an intervention trial [38]. These potential benefits should be considered in light of the differences between the contemporary PD and the traditional PD of our ancestors. For instance, there is ample evidence supporting a high intake of animal protein in the traditional PD [39], whilst animal protein originating from lean meats and seafood constitutes the majority of energy intake in contemporary PDs [30]. Moreover, compared to contemporary whole-food diets, such as the Mediterranean diet, the superiority of PDs could be attributed to the restriction of grains [40].
Potential mechanisms by which the PD prevents BC include the following. First, the PD limits processed and sugar-laden foods, which are deleterious through pathways of oxidative stress and inflammation [41,42,43]. Moreover, it has been suggested that the PD pattern increases insulin sensitivity [31, 44], a mechanism linked to lower BC risk [45]. Second, the PD is based on good sources of fibre, antioxidants, and unsaturated fatty acids that beneficially modulate detoxification enzymes and the immune system [46,47,48]. Fibre is suggested to reduce BC risk through effects on the discharge of carcinogens in the gut, promotion of probiotics, absorption of free oestrogen, and beneficial effects on insulin resistance [49,50,51]. Third, the removal of grains from the diet is postulated to reduce inflammation and benefit hormone levels [52, 53]. Fourth, the PD limits non-lean red and processed meat linked to oxidative stress and systemic inflammation [54,55,56], also suggested by lower levels of inflammatory biomarkers in an observational study [35]. Fifth, a link between pathways of obesity-linked inflammation and cancer risk is probable, given that PD has shown beneficial effects in controlling weight gain [57, 58]. Lastly, sodium restriction may have beneficial mechanistic effects on cancer pathophysiology [59].
This study has some important strengths—the prospective nature and the large sample size with high retention and a long follow-up period. There was a large number of cases with documented receptor and histological subtypes. Excluding participants with BC diagnosed in the first five years of follow-up did not change our results, suggesting that reverse causation was unlikely to explain our findings.
However, this study has some limitations. First, the modern PD is likely different in terms of the nutritional value of the diet of our preagricultural ancestors. Second, as with other dietary pattern analyses, participants did not explicitly decide to adhere to the PD but were following a dietary pattern which was more or less similar to the PD definition. Third, two dietary assessments over the long follow-up may not capture optimally dietary changes in the interim period between FFQs. We used the cumulative average dietary score for participants with long follow-up to account for this limitation. Fourth, there could be some degree of non-differential misclassification in the dietary assessment, which might have biased results towards the null. Lastly, highly educated participants may not represent the general French population, limiting the external validity of our results. However, stronger associations might be expected considering the general population’s dietary variations.
Conclusions
In conclusion, higher adherence to the PD was associated with a 17% lower BC risk among postmenopausal women, which could translate into a substantial impact on the number of avoided cases of cancer so common. These findings support the long-term healthful influence of the PD, based on lean meat, fish, fruit, vegetables, and nuts, with the limitation of dairy, grains, legumes, refined sugar, and alcohol. More studies are needed to confirm the findings and understand the underlying mechanistic associations.
Data availability
The datasets generated during and/or analysed for the current study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Azamjah N, Soltan-Zadeh Y, Zayeri F. Global trend of breast cancer mortality rate: a 25-year study. Asian Pac J Cancer Prev. 2019;20:2015–20.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Fitzmaurice C. Global Burden of Disease Cancer C. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 2006 to 2016: A systematic analysis for the Global Burden of Disease study. J Clin Oncol. 2018;36:1553–68.
Sharma R. Breast cancer incidence, mortality and mortality-to-incidence ratio (MIR) are associated with human development, 1990-2016: evidence from Global Burden of Disease Study 2016. Breast Cancer. 2019;26:428–45.
Institute of M, National Research Council National Cancer Policy B. Fulfilling the Potential of Cancer Prevention and Early Detection. Washington (DC): National Academies Press (US); 2014 2014/07/25/.
Ghaedi E, Mohammadi M, Mohammadi H, Ramezani-Jolfaie N, Malekzadeh J, Hosseinzadeh M, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634–46.
Jönsson T, Granfeldt Y, Ahrén B, Branell U, Pålsson G, Hansson A. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
Manheimer EW, van Zuuren EJ, Fedorowicz Z, Pijl H. Paleolithic nutrition for metabolic syndrome: systematic review and meta-analysis. Am J Clin Nutr. 2015;102:922–32.
Whalen KA, McCullough M, Flanders WD, Hartman TJ, Judd S, Bostick RM. Paleolithic and mediterranean diet pattern scores and risk of incident, sporadic colorectal adenomas. Am J Epidemiol. 2014;180:1088–97.
Kushi LH, Potter JD, Bostick RM, Drinkard CR, Sellers TA, Gapstur SM, et al. Dietary fat and risk of breast cancer according to hormone receptor status. Cancer Epidemiol Biomark Prev. 1995;4:11–9.
Olsen A, Tjonneland A, Thomsen BL, Loft S, Stripp C, Overvad K, et al. Fruits and vegetables intake differentially affects estrogen receptor negative and positive breast cancer incidence rates. J Nutr. 2003;133:2342–7.
Zhang SM, Hankinson SE, Hunter DJ, Giovannucci EL, Colditz GA, Willett WC. Folate intake and risk of breast cancer characterized by hormone receptor status. Cancer Epidemiol Biomark Prev. 2005;14:2004–8.
Fung TT, Hu FB, Holmes MD, Rosner BA, Hunter DJ, Colditz GA, et al. Dietary patterns and the risk of postmenopausal breast cancer. Int J Cancer. 2005;116:116–21.
Shin S, Saito E, Inoue M, Sawada N, Ishihara J, Takachi R, et al. Dietary pattern and breast cancer risk in Japanese women: the Japan Public Health Center-based Prospective Study (JPHC Study). Br J Nutr. 2016;115:1769–79.
Velie EM, Schairer C, Flood A, He J-P, Khattree R, Schatzkin A. Empirically derived dietary patterns and risk of postmenopausal breast cancer in a large prospective cohort study. Am J Clin Nutr. 2005;82:1308–19.
Mannisto S, Harald K, Harkanen T, Maukonen M, Eriksson JG, Heikkinen S, et al. Association between overall diet quality and postmenopausal breast cancer risk in five Finnish cohort studies. Sci Rep. 2021;11:16718.
Haridass V, Ziogas A, Neuhausen SL, Anton-Culver H, Odegaard AO. Diet quality scores inversely associated with postmenopausal breast cancer risk are not associated with premenopausal breast cancer risk in the California teachers study. J Nutr. 2018;148:1830–7.
Clavel-Chapelon F. Cohort profile: the French E3N cohort study. Int J Epidemiol. 2015;44:801–9.
Cottet V, Touvier M, Fournier A, Touillaud MS, Lafay L, Clavel-Chapelon F, et al. Postmenopausal breast cancer risk and dietary patterns in the E3N-EPIC prospective cohort study. Am J Epidemiol. 2009;170:1257–67.
Lucas F, Niravong M, Villeminot S, Kaaks R, Clavel-Chapelon F. Estimation of food portion size using photographs: validity, strengths, weaknesses and recommendations. J Hum Nutr Dietetics. 1995;8:65–74.
van Liere M. Relative validity and reproducibility of a French dietary history questionnaire. Int J Epidemiol. 1997;26:128S–36.
Jean-Claude F, Jayne I, Carole T, Max F. Répertoire général des aliments - Table de composition. 2nd ed. Paris (FRA):Inra;1995.
Food and Agriculture Organization of the United Nations. Guidelines for measuring household and individual dietary diversity 2013. Available from: https://www.fao.org/3/i1983e/i1983e.pdf.
Shah S, MacDonald CJ, El Fatouhi D, Mahamat-Saleh Y, Mancini FR, Fagherazzi G, et al. The associations of the Palaeolithic diet alone and in combination with lifestyle factors with type 2 diabetes and hypertension risks in women in the E3N prospective cohort. Eur J Nutr. 2021;60:3935–45.
Tehard B, van Liere MJ, Com Nougue C, Clavel-Chapelon F. Anthropometric measurements and body silhouette of women: validity and perception. J Am Diet Assoc. 2002;102:1779–84.
Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis: Springer; 2013.
Allison PD. Survival Analysis Using SAS®: A Practical Guide, Second Edition. Cary, NC: SAS Institute Inc. 2010.
Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112:580–93.
Cordain L. The nutritional characteristics of a contemporary diet based upon Paleolithic food groups. J Am Nutraceutical Assoc. 2002;5:15–24.
Frassetto LA, Schloetter M, Mietus-Synder M, Morris RC Jr, Sebastian A. Metabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type diet. Eur J Clin Nutr. 2009;63:947–55.
Osterdahl M, Kocturk T, Koochek A, Wandell PE. Effects of a short-term intervention with a paleolithic diet in healthy volunteers. Eur J Clin Nutr. 2008;62:682–5.
Bisht B, Darling WG, Grossmann RE, Shivapour ET, Lutgendorf SK, Snetselaar LG, et al. A multimodal intervention for patients with secondary progressive multiple sclerosis: feasibility and effect on fatigue. J Alter Complement Med. 2014;20:347–55.
Bligh HF, Godsland IF, Frost G, Hunter KJ, Murray P, MacAulay K, et al. Plant-rich mixed meals based on Palaeolithic diet principles have a dramatic impact on incretin, peptide YY and satiety response, but show little effect on glucose and insulin homeostasis: an acute-effects randomised study. Br J Nutr. 2015;113:574–84.
Whalen KA, McCullough ML, Dana Flanders W, Hartman TJ, Judd S, Bostick RM. Paleolithic and mediterranean diet pattern scores are inversely associated with biomarkers of inflammation and oxidative balance in adults. J Nutr. 2016;146:1217–26.
Whalen K, Judd S, McCullough M, Flanders W, Hartman T, Bostick R. Paleolithic and mediterranean diet pattern scores are inversely associated with all-cause and cause-specific mortality in adults. J Nutr. 2017;147:612–20.
Jospe MR, Roy M, Brown RC, Haszard JJ, Meredith-Jones K, Fangupo LJ, et al. Intermittent fasting, Paleolithic, or Mediterranean diets in the real world: exploratory secondary analyses of a weight-loss trial that included choice of diet and exercise. Am J Clin Nutr. 2020;111:503–14.
Klement RJ, Koebrunner PS, Krage K, Weigel MM, Sweeney RA. Short-term effects of a Paleolithic lifestyle intervention in breast cancer patients undergoing radiotherapy: a pilot and feasibility study. Med Oncol. 2020;38:1.
Ben-Dor M, Sirtoli R, Barkai R. The evolution of the human trophic level during the Pleistocene. Am J Phys Anthropol. 2021;175:27–56.
Spreadbury I. Comparison with ancestral diets suggests dense acellular carbohydrates promote an inflammatory microbiota, and may be the primary dietary cause of leptin resistance and obesity. Diabetes Metab Syndr Obes. 2012;5:175–89.
Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–16.
Debras C, Chazelas E, Srour B, Kesse-Guyot E, Julia C, Zelek L, et al. Total and added sugar intakes, sugar types, and cancer risk: results from the prospective NutriNet-Sante cohort. Am J Clin Nutr. 2020;112:1267–79.
Fiolet T, Srour B, Sellem L, Kesse-Guyot E, Alles B, Mejean C, et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Sante prospective cohort. BMJ. 2018;360:k322.
Otten J, Stomby A, Waling M, Isaksson A, Tellstrom A, Lundin-Olsson L, et al. Benefits of a Paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:e2828.
Stoll BA. Western nutrition and the insulin resistance syndrome: a link to breast cancer. Eur J Clin Nutr. 1999;53:83–7.
Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, et al. Association of nut consumption with total and cause-specific mortality. N. Engl J Med. 2013;369:2001–11.
Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am J Clin Nutr. 1999;70:475S–90S.
Radzikowska U, Rinaldi AO, Celebi Sozener Z, Karaguzel D, Wojcik M, Cypryk K, et al. The influence of dietary fatty acids on immune responses. Nutrients. 2019;11:2990.
Chen S, Chen Y, Ma S, Zheng R, Zhao P, Zhang L, et al. Dietary fibre intake and risk of breast cancer: A systematic review and meta-analysis of epidemiological studies. Oncotarget. 2016;7:80980–9.
Dallal CM, Lacey JV Jr, Pfeiffer RM, Bauer DC, Falk RT, Buist DS, et al. Estrogen metabolism and risk of postmenopausal endometrial and ovarian cancer: the B approximately FIT cohort. Horm Cancer. 2016;7:49–64.
Cummings JH, Mann JI, Nishida C, Vorster HH. Dietary fibre: an agreed definition. Lancet. 2009;373:365–6.
de Punder K, Pruimboom L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients. 2013;5:771–87.
Cordain L. Cereal grains: humanity’s double-edged sword. World Rev Nutr Diet. 1999;84:19–73.
McAfee AJ, McSorley EM, Cuskelly GJ, Moss BW, Wallace JMW, Bonham MP, et al. Red meat consumption: an overview of the risks and benefits. Meat Sci. 2010;84:1–13.
Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer. 2008;60:131–44.
Ward MH, Cross AJ, Divan H, Kulldorff M, Nowell-Kadlubar S, Kadlubar FF, et al. Processed meat intake, CYP2A6 activity and risk of colorectal adenoma. Carcinogenesis. 2007;28:1210–6.
de Menezes EVA, Sampaio HAC, Carioca AAF, Parente NA, Brito FO, Moreira TMM, et al. Influence of Paleolithic diet on anthropometric markers in chronic diseases: systematic review and meta-analysis. Nutr J. 2019;18:41.
Blomquist C, Chorell E, Ryberg M, Mellberg C, Worrsjö E, Makoveichuk E, et al. Decreased lipogenesis-promoting factors in adipose tissue in postmenopausal women with overweight on a Paleolithic-type diet. Eur J Nutr. 2018;57:2877–86.
Abdoli A. High salt and fat intake, inflammation, and risk of cancer. Front Biol. 2018;12:387–91.
Acknowledgements
The research was carried out using data from INSERM (French National Institutes for Health and Medical Research) E3N cohort, which was established and maintained with the support of the Mutuelle Générale de l’Education Nationale (MGEN), Gustave Roussy, and the French League against Cancer (LNCC). E3N-E4N is also supported by the French National Research Agency (ANR) under the Investment for the Future Programme (PIA) (ANR-10-COHO-0006) and by the French Ministry of Higher Education, Research and Innovation (subsidy for public service charges n°2103 586016). The authors are indebted to all participants for their continued participation. They are also grateful to all members of the E3N study group.
Funding
This research was carried out using data from INSERM’S E3N cohort with the support of the MGEN, Institut Gustave Roussy and the “Ligue contre le Cancer” for the constitution and maintenance of the E3N cohort. This work has also benefited from State aid managed by the National Research Agency under the programme “Investissement d’avenir” under the reference ANR-10-COHO-0006 as well as a subsidy from the “Ministère de l’enseignement supérieur de la recherche et de l’innovation” for public service charges under the reference n°2103 586016. SS is supported by a doctoral funding from l’Ecole Doctorale de Santé Publique, Ministère de l’enseignement supérieur, de la recherche et de l’innovation.
Author information
Authors and Affiliations
Contributions
SS, MCBR, and NL conceived and designed the study. MCBR and NL contributed equally as the last authors. SS performed the statistical analysis and drafted the original manuscript. All authors contributed to the interpretation of data discussed in the manuscript, revised it, and approved its final version to be published. NL is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study participants provided written informed consent, and the cohort study received ethical approval from the French National Commission for Computerized Data and Individual Freedom.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, S., Mahamat-Saleh, Y., Hajji-Louati, M. et al. Palaeolithic diet score and risk of breast cancer among postmenopausal women overall and by hormone receptor and histologic subtypes. Eur J Clin Nutr 77, 596–602 (2023). https://doi.org/10.1038/s41430-023-01267-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-023-01267-x
This article is cited by
-
Inverse association between Paleolithic Diet Fraction and mortality and incidence of cardiometabolic disease in the prospective Malmö Diet and Cancer Study
European Journal of Nutrition (2024)
-
Adherence to the Paleolithic diet and Paleolithic-like lifestyle reduce the risk of colorectal cancer in the United States: a prospective cohort study
Journal of Translational Medicine (2023)