Abstract
Objectives
We aimed to evaluate the inter-hospital variability of gestational weight gain (GWG) among women with gestational diabetes mellitus (GDM) in China and explore GDM-specific optimal GWG relative to the National Academy of Medicine (NAM) targets.
Methods
A prospective multicenter University Hospital Advanced Age Pregnant Cohort study was conducted from March 2017 to June 2021 at eight hospitals in China. The range of mean GWG across hospitals and the intraclass correlation coefficient (ICC) were used to evaluate the inter-hospital variability of GWG. For normal-weight and overweight women with GDM, potential optimal GWGs were derived by minimizing the joint risk of small and large for gestational age (SGA and LGA), and the incidences of adverse perinatal outcomes were compared between women who met the optimal GWGs and those who met the NAM targets.
Results
A total of 3,013 women with GDM and 9,115 women without GDM were included. The GWG variation among hospitals was larger in women with GDM (range: 10.0–14.1 kg, ICC = 7.1%) than in women without GDM (range: 13.0–14.5 kg, ICC = 0.7%). The estimated optimal GWGs for women with GDM were lower than the NAM targets, as 9.5–14.0 kg for normal-weight and 3.0–7.5 kg for overweight women. Women with GDM who met the optimal GWGs had lower incidences of LGA and macrosomia compared to those who met the NAM targets, with no significant increase in the incidences of SGA, preterm birth, etc.
Conclusions
The marked variation of GWG among hospitals in women with GDM indicates the need to develop optimal GWGs for them. The potential optimal GWGs for women with GDM might be lower than the NAM targets, likely benefiting the perinatal outcomes.
Similar content being viewed by others
Introduction
Gestational diabetes mellitus (GDM), a common condition involving glucose intolerance that onsets or is first recognized during pregnancy [1], increases the risks of adverse perinatal outcomes such as macrosomia and neonatal hypoglycemia [2, 3], and the future risk of type 2 diabetes for the mother and offspring [4, 5]. GDM is prevalent in many countries [6]. The worldwide incidence was 13.2% in 2019, affecting approximately 17.1 million births [7], and the corresponding values in China were 14.8% and 2.2 million births [8]. The incidence is particularly high in women of advanced maternal age (AMA), reaching 27% [8].
Gestational weight gain (GWG), usually defined as a change in maternal weight measured before pregnancy and prior to delivery, is associated with the short- and long-term health of the mother and offspring [9]. GWG in women with GDM is particularly concerning because GWG affects the occurrence and prognosis of GDM [10] and has a synergistic effect with GDM on perinatal outcomes [11]. To improve glycemic control, physical activities and dietary modifications are generally advocated for women with GDM [12], likely leading to a lower GWG than women without GDM [13]. Nevertheless, due to the lack of GWG recommendations for women with GDM, their GWG might vary among hospitals [14]. Thus far, no studies have evaluated the inter-hospital variability of GWG in women with GDM.
Whether the National Academy of Medicine (NAM) targets are applicable to women with GDM remains unclear [15]. Some previous studies reported that a GWG below the NAM targets in women with GDM improved glycemic control [16], decreased the risks of large for gestational age (LGA) and macrosomia [14], without increasing the risk of small for gestational age (SGA) or preterm birth [14, 17,18,19], indicating that the potential optimal GWGs for women with GDM might be lower than the NAM targets. However, to what extent the NAM targets should be left-shifted has not been determined. Two studies assessed the effects of GWG, subtracting 1–2 kg from the NAM targets, on perinatal outcomes in women with GDM but reported inconsistent results [20, 21]. In addition, neither of them considered the effects of pre-pregnancy body mass index (BMI).
In this prospective multicenter cohort study, we aimed to evaluate the inter-hospital variability of GWG among women with GDM in China, and to explore the GDM-specific optimal GWG relative to the NAM targets according to maternal pre-pregnancy BMI.
Methods
Cohort and study participants
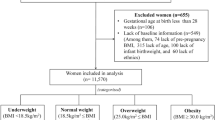
The data were drawn from the prospective multicenter University Hospital Advanced Age Pregnant (UNIHOPE) Cohort study conducted in China from March 2017 to June 2021 [22]. The cohort comprised a singleton and a twin pregnancy subcohorts. This study was based on the singleton pregnancy subcohort, which encompassed nine tertiary hospitals in the cities of Beijing, Shanghai, Guangzhou, Shenyang, Wuhan, Chongqing, and Chengdu, covering the eastern, central, and western regions of China. For this subcohort, the inclusion criteria were women of AMA planning to receive prenatal healthcare and delivery service at the study hospitals. Additionally, some younger women (≤35 years; proportion of 25%) were recruited for potential comparisons between the two populations. The exclusion criteria were women with no ability to provide informed consent or with mental disorders. The participants were enrolled and completed early pregnancy follow-up before 14 gestational weeks, completed mid-pregnancy follow-up at 24 to 28 gestational weeks, and provided delivery information after delivery and before discharge.
During the cohort period, 15,597 pregnant women at the nine hospitals who delivered a live birth were initially considered in this study. The exclusion criteria were (1) pregnant women at the hospital with enrollment size <200 (n = 105); (2) missing maternal baseline characteristics or maternal age <20 or >50 years (n = 563); (3) missing delivery information, gestational age at delivery <24 or >44 weeks, or birth weight <1000 or >5000 g (n = 443); (4) missing (n = 1,396) or suspicious (n = 296; defined as the value exceeding the range of median ± 3 interquartile ranges [IQRs]) maternal height, weight, or GWG; and (5) diagnosis of pre-gestational diabetes (n = 662) or missing diabetes diagnosis (n = 4). Finally, 12,128 pregnant women at eight hospitals were included in the analysis, consisting of 3,013 women with GDM and 9,115 women without GDM. In analysis of GWG after OGTT, women who delivered before 28 gestational weeks (n = 12), or with missing (n = 1978) or suspicious (n = 300) weight or GWG in the early or mid-pregnancy were further excluded, leaving 2,611 women with GDM and 7227 women without GDM included. The maternal characteristics between the excluded and the included women were similar except for maternal age, ethnicity, parity, and pre-pregnancy BMI, as detailed in our previous study [23].
Data collection and definitions
Information about maternal demographics, reproductive history, prenatal care of the current pregnancy, and perinatal outcomes was collected using a structured questionnaire by nurses or obstetricians. According to the criteria of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) [24], GDM was diagnosed when a 75 g oral glucose tolerance test (OGTT) at 24 to 28 gestational weeks met one of the following three criteria: (1) fasting plasma glucose ≥ 5.1 mmol/L, (2) 1 h plasma glucose ≥10.0 mmol/L, or (3) 2 h plasma glucose ≥ 8.5 mmol/L.
The pre-pregnancy weight was self-reported at enrollment; the pre-delivery weight and height were measured at the hospital. The median difference between the date of the pre-delivery weight measurement and the delivery date was one day. The pre-pregnancy BMI (kg/m2) was calculated as the pre-pregnancy weight divided by the height squared and categorized as underweight (<18.5 kg/m2), normal-weight (18.5 to <24.0 kg/m2), overweight (24.0 to <28.0 kg/m2), or obese (≥28.0 kg/m2) according to the Chinese criteria [25], which were recommended when applying the NAM targets to Chinese women [26]. The total GWG (kg) was calculated as the pre-delivery weight minus the pre-pregnancy weight and categorized as insufficient, adequate, or excessive according to the NAM targets [15]. As OGTT was performed at the end of mid-pregnancy, GWG after OGTT was approximate to the GWG during late pregnancy calculated as pre-delivery weight minus weight at the 27th gestational week. The weekly GWG after OGTT was calculated as the GWG after OGTT divided by 12 gestational weeks (the median gestational age at delivery [39 weeks] minus 27 weeks). If the mid-pregnancy follow-up did not occur at the 27th gestational week, a linear interpolation method was used to estimate the weight [27].
Adverse perinatal outcomes included SGA, LGA, macrosomia, preterm birth, neonatal intensive care unit (NICU) admission, cesarean delivery, postpartum hemorrhage (PPH), and gestational hypertensive disorders (GHDs). SGA was defined as birth weight <10th percentile for gestational age and LGA as birth weight > 90th percentile for gestational age, according to the Chinese sex- and gestational week-specific birth weight standards [28, 29]. Macrosomia was defined as birth weight ≥4000 g, preterm birth as birth with gestational age at delivery <37 weeks, PPH as blood loss >500 mL for vaginal delivery or >1000 mL for cesarean delivery, and GHDs as new-onset systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg after 20 gestational weeks.
Statistical analyses
The continuous variables were expressed as means ± standard deviations (SDs) or medians (IQRs), depending on the normality of the data distribution. The categorical variables were expressed as frequencies (%).
The range of mean GWG across the eight hospitals and the intraclass correlation coefficient (ICC) derived from a random intercept model were used to evaluate the inter-hospital variability of GWG [30]. The least squares mean [31] and conditional ICC [32] were further calculated to account for the differences in maternal characteristics among hospitals.
The potential optimal GWGs for women with GDM were developed by the method of minimizing the joint risk of SGA and LGA, which has been used previously in generating GWG targets for general pregnant women [33, 34]. The potential optimal GWGs were developed for normal-weight (n = 1,919) and overweight women (n = 747), but not for underweight (n = 163) or obese women (n = 184) because of their limited sample sizes. The adjusted predictive probabilities of SGA and LGA with respect to GWG were estimated by mixed-effects logistic regression with the marginal standardization method [35]. The potential optimal GWG point was the GWG with the lowest sum of the predictive probability of SGA and LGA. The difference between the potential optimal GWG point and the midpoint of the NAM range was calculated, and then the potential optimal GWGs were obtained by subtracting the difference from both the NAM upper and lower limits. Alternatively, the potential optimal GWGs were estimated as the GWG range with the sum of predictive probabilities increasing no more than 0.5% from the potential optimal GWG point [34]. The maximum increase of 0.5% was selected to avoid excessively wide potential optimal GWGs [33]. The adjusted covariates included maternal age, ethnicity, parity, conception mode, and gestational age. Center effects were adjusted by including random effects of centers. To obtain the potential optimal GWGs for the subgroup of AMA and younger women, the mixed-effects logistic regression with the prediction at the modes method was used [35], fixing the maternal age at ≥35 years and <35 years, respectively. For facilitating clinical use, the potential optimal GWG and weekly GWG after OGTT were also estimated.
Mixed-effects logistic regression was performed to estimate the adjusted absolute risk reduction (ARR) [36], to compare the incidences of the adverse perinatal outcomes between women who met the potential optimal GWGs and those who met the NAM targets. The incidences of the adverse outcomes were additionally compared between women who met the potential optimal GWGs only and those who met the NAM targets only, with those who met both excluded.
Statistical analyses were performed using R software (version 4.0; R Development Core Team, Vienna, Austria). Statistical tests were two-sided, with the significance level set at 0.05.
Results
Characteristics of study participants
The characteristics of the women with and without GDM are shown in Table 1. Women with versus without GDM were more likely to be of AMA (89.6% vs. 78.4%), to undergo in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) (18.0% vs. 12.5%), to have a higher pre-pregnancy BMI (22.8 ± 3.0 vs. 21.8 ± 2.8 kg/m2), to give birth by cesarean delivery (68.6% vs. 63.7%), and to deliver preterm births (12.2% vs. 9.0%) with a higher incidence of NICU admission (7.1% vs. 5.4%). Although women with versus without GDM were more likely to deliver lower weight births (3231.0 ± 511.0 vs. 3262.0 ± 482.0 g), the incidences of LGA, SGA, and macrosomia were similar between the two groups. For women with GDM, 15.3% were treated with insulin, and the rest were treated with nutritional diet and/or physical exercise.
Inter-hospital variability of GWG
The GWG was 11.4 ± 5.1 kg and 13.5 ± 5.0 kg in women with and without GDM, respectively. The mean GWG across the eight hospitals ranged from 10.0 to 14.1 kg in women with GDM, and from 13.0 to 14.5 kg in women without GDM, with corresponding ICCs of 7.1% and 0.7%, respectively. After adjustment for maternal characteristics, the least squares mean of GWG across the eight hospitals ranged from 10.7 to 14.8 kg in women with GDM, and from 13.1 to 14.8 kg in women without GDM, with corresponding conditional ICCs of 7.0% and 0.6%, respectively (Supplemental Table 1).
Potential optimal GWGs for women with GDM
The predictive probability curves of SGA, LGA, and sum of SGA and LGA (SGA + LGA) with respect to GWG for normal-weight and overweight women with GDM are shown in Fig. 1. The predictive probability of SGA + LGA was lowest at 11.7 kg of GWG in normal-weight and 5.2 kg in overweight women. Correspondingly, the potential optimal GWGs were 9.5–14.0 kg for normal-weight and 3.0–7.5 kg for overweight women. The results were similar when the optimal GWGs were estimated as the GWG range with the sum of predictive probabilities increasing no more than 0.5% from the optimal GWG point, as 9.1–14.3 kg for normal-weight and 2.7–7.6 kg for overweight women (Table 2). The potential optimal GWGs were slightly lower in women of AMA than in younger women (Supplemental Table 2 and Supplemental Fig. 1).
Predictive probability was estimated by mixed-effects logistic regression with the marginal standardization method. The area within the two vertical dashed lines corresponds to the NAM targets, the shading to the potential optimal GWGs (the primary method), and the triangle to the lowest predictive probability of SGA + LGA.
Compared to women with GDM who met the NAM targets, those who met the potential optimal GWGs had lower incidences of LGA (adjusted ARR = − 2.8%, 95% CI: − 5.2% to −0.3%) and macrosomia (adjusted ARR = − 3.0%, 95% CI: − 4.8% to −1.1%), with no significant differences in the incidences of SGA, preterm birth, NICU admission, cesarean delivery, PPH, or GHDs. In subgroup analyses, the decreased incidence of macrosomia was consistently significant in normal-weight (adjusted ARR = −2.5%, 95% CI: −4.5% to −0.5%) and overweight women (adjusted ARR = −4.4%, 95% CI: −8.0% to −0.7%), as was the decreased incidence of LGA in overweight women (adjusted ARR = −5.3%, 95% CI: −9.9% to −0.6%). The results remained when the comparisons were performed between women who met the potential optimal GWGs only and those who met the NAM targets only, with those who met both excluded (Table 3).
Potential optimal GWG after OGTT for women with GDM
The potential optimal GWG and weekly GWG after OGTT according to GWG before OGTT are shown in Supplemental Table 3. The potential optimal GWG after OGTT decreased with the increasing of GWG before OGTT. When the GWG before OGTT was ≥10.0 kg for normal-weight or ≥5.0 kg for overweight women, the potential optimal GWG after OGTT was negative.
Discussion
In this prospective multicenter cohort study in China, the GWG of women with GDM varied more markedly among hospitals than that of women without GDM. The potential optimal GWGs for women with GDM were lower than the NAM targets, as 9.5–14.0 kg for normal-weight and 3.0–7.5 kg for overweight women. Women with GDM who met the potential optimal GWGs had lower incidences of LGA and macrosomia, without significant increase in the incidences of SGA, preterm birth, NICU admission, cesarean delivery, PPH, or GHDs, as compared to those who met the NAM targets.
This is, to our knowledge, the first report of a higher inter-hospital variability of GWG in women with than without GDM. For example, the hospital factors accounted for 7.1% of the GWG variation in women with GDM, compared to 0.7% in women without GDM (ICC: 7.1% vs. 0.7%) [30]. The results remained after adjustment for maternal characteristics, indicating that the marked variation of GWG in women with GDM is likely due to the varied weight-gain management strategies among hospitals, necessitating the development of GDM-specific optimal GWGs.
Few studies have attempted to determine optimal GWGs for women with GDM [20, 21, 37]. The study by Wu et al. among 1,820 Chinese women with GDM tried to develop optimal GWG rates during the second and third trimesters, but the rates in all pre-pregnancy BMI categories were higher than the NAM recommendations [37], which would possibly jeopardize the glycemic control and birth weight outcomes [16]. Another two studies examined the effects of a lower GWG, subtracting 1–2 kg from the NAM targets, on perinatal outcomes, but reported inconsistent results [20, 21]. The study by Wong et al. among 2638 Australian women with GDM found that the lower GWG did not improve perinatal outcomes [21], while the study by Xu et al. among 1200 Chinese women with GDM found that the lower GWG was associated with decreased risks of LGA and macrosomia, with a slightly increased risk of SGA [20]. The lower GWG in these two studies was somewhat subjective, and neither study took account of the pre-pregnancy BMI, a potential modifier for the association of GWG with perinatal outcomes [38]. We estimated the potential optimal GWGs by minimizing the joint risk of SGA and LGA for normal-weight and overweight women with GDM, and found that subtracting 2.0 kg from the NAM targets for normal-weight and 4.0 kg for overweight women might be more reasonable. We also found that if the GWG before OGTT was excessive, weight loss after OGTT might be recommended, similar to previous studies [10, 39].
In our study, the potential optimal GWGs compared to the NAM targets in women with GDM decreased the risks of LGA and macrosomia, in line with previous studies [40, 41]. A lower GWG contributes to controlling maternal hyperglycemia and excessive fetal growth [10]. We also found that the potential optimal GWGs did not increase the risk of SGA or preterm birth, consistent with a meta-analysis which found that a GWG below the NAM targets among women with GDM did not increase the risk of SGA or preterm birth [14]. This might relate to that a lower GWG in women with GDM could reduce the risk of GHDs [42], a risk factor for SGA and preterm birth [43]. In our study, the incidence of GHDs tended to decrease by 2.5 percentage points in women who met the potential optimal GWGs compared to those who met the NAM targets (adjusted ARR = −2.5%, P = 0.08). In addition, perhaps women with a lower GWG were more likely to accept nutritional diet interventions, have better diet quality, and obtain more attention from doctors, preventing SGA and preterm birth [18, 44, 45].
This study had strengths. Using data from a prospective multicenter cohort in China, we firstly reported a higher inter-hospital variability of GWG in women with than without GDM, possibly reflecting the varied weight-gain management strategies in clinical practice due to the lack of GWG recommendations for women with GDM. Based on the modeling strategies that have been used previously [33, 34], we developed potential optimal GWGs for normal-weight and overweight women with GDM, which is crucial to prenatal care providers and clinicians, particularly given the increasing prevalence of GDM [46].
This study also had limitations. First, nearly 90% of women with GDM in our study were of AMA, thus caution is needed when generalizing the potential optimal GWGs to younger women, although our data showed comparable optimal GWGs between the two populations. Additionally, the potential optimal GWGs were estimated from Chinese women, and might not be applicable to other ethnic women, as Chinese women with GDM were less likely to have adverse perinatal outcomes, different from Caucasian women [47]. Second, despite the larger sample size than the previous studies [20, 21, 37], the potential optimal GWGs were only explored for normal-weight and overweight women but not for underweight or obese women, considering that the small number of participants would lead to unstable results. Third, potential bias might be introduced as 17% of participants were excluded due to missing or suspicious data, despite the similar characteristics between the included and excluded women. Fourth, potential bias might also be introduced by the self-reported pre-pregnancy weight, despite a strong positive correlation between self-reported and measured pre-pregnancy weight [48].
In conclusion, the marked variation of GWG in women with GDM likely reflects varied weight-gain management strategies among hospitals in China, necessitating the development of optimal GWGs. The potential optimal GWGs, lower than the NAM targets, likely benefit the perinatal outcomes. Nevertheless, it should be noted that the potential optimal GWGs in our study were developed for ordinary women with GDM; special groups such as those with other pregnancy complications should cautiously use these targets. The potential optimal GWGs should also be examined in larger and more representative samples, including younger, underweight, and obese women.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Buchanan TA, Xiang A, Kjos SL, Watanabe R. What is gestational diabetes? Diabetes Care. 2007;30:S105–11.
Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66:14–20.
Thevarajah A, Simmons D. Risk factors and outcomes for neonatal hypoglycaemia and neonatal hyperbilirubinaemia in pregnancies complicated by gestational diabetes mellitus: a single centre retrospective 3-year review. Diabet Med. 2019;36:1109–17.
Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361.
Scholtens DM, Kuang A, Lowe LP, Hamilton J, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care. 2019;42:381–92.
Zhu YY, Zhang CL. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16:7.
International Diabetes Federation. IDF Diabetes Atlas 9th edition. 2019; https://diabetesatlas.org/atlas/ninth-edition/.
Gao CH, Sun X, Lu L, Liu FW, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta‐analysis. J Diabetes Investig. 2019;10:154–62.
Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317:2207–25.
Barnes RA, Wong T, Ross GP, Griffiths MM, Smart CE, Collins CE, et al. Excessive weight gain before and during gestational diabetes mellitus management: what is the impact? Diabetes Care. 2020;43:74–81.
Black MH, Sacks DA, Xiang AH, Lawrence JM. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diabetes Care. 2013;36:56–62.
Juan J, Yang HX. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int J Environ Res Pub Health. 2020;17:9517.
Stewart ZA, Wallace EM, Allan CA. Patterns of weight gain in pregnant women with and without gestational diabetes mellitus: An observational study. Aust N. Z J Obstet Gynaecol. 2012;52:433–9.
Viecceli C, Remonti LR, Hirakata VN, Mastella LS, Gnielka V, Oppermann MLR, et al. Weight gain adequacy and pregnancy outcomes in gestational diabetes: a meta-analysis. Obes Rev. 2017;18:567–80.
IOM (Institute of Medicine). Weight gain during pregnancy: reexamining the guidelines. National Academies Press: Washington, DC, 2009.
Cheng YW, Chung JH, Kurbisch-Block I, Inturrisi M, Shafer S, Caughey AB. Gestational weight gain and gestational diabetes mellitus: perinatal outcomes. Obstet Gynecol. 2008;112:1015–22.
Mastella LS, Weinert LS, Gnielka V, Hirakata VN, Oppermann MLR, Silveiro SP, et al. Influence of maternal weight gain on birth weight: a gestational diabetes cohort. Arch Endocrinol Metab. 2018;62:55–63.
Bogdanet D, Mustafa M, Khattak A, O’Shea PM, Dunne FP. Atlantic DIP: is weight gain less than that recommended by IOM safe in obese women with gestational diabetes mellitus? Int J Obes. 2021;45:1044–51.
Bogdanet D, O’shea PM, Dunne FP. Is gestational weight gain below that recommended by IOM for obese women with insulin treated gestational diabetes safe? Diabetes. 2018;67:S1456-P.
Xu QY, Ge ZJ, Hu J, Shen SM, Bi Y, Zhu DL. The association of gestational weight gain and adverse pregnancy outcomes in women with gestational diabetes mellitus. Endocr Pr. 2019;25:1137–50.
Wong T, Barnes RA, Ross GP, Cheung NW, Flack JR. Are the Institute of Medicine weight gain targets applicable in women with gestational diabetes mellitus? Diabetologia. 2017;60:416–23.
Liu JM University Hospital Advanced Age Pregnant Cohort (UNIHOPE). 2017; https://clinicaltrials.gov/ct2/show/NCT03220750.
Cheng ZH, Wei YM, Li HT, Yu HZ, Liu JM, Zhou YB. Gestational diabetes mellitus as an effect modifier of the association of gestational weight gain with perinatal outcomes: A prospective cohort study in China. Int J Environ Res Public Health. 2022;19:5615.
International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82.
Zhou BF. Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults-study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96.
Goldstein RF, Abell SK, Ranasinha S, Misso ML, Boyle JA, Harrison CL, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018;16:153.
Hu JJ, Aris IM, Oken E, Ma YA, Ding N, Gao M, et al. Association of total and trimester-specific gestational weight gain rate with early infancy weight status: a prospective birth cohort study in China. Nutrients 2019;11:280.
Zhu L, Zhang R, Zhang SL, Shi WJ, Yan WL, Wang XL, et al. Chinese neonatal birth weight curve for different gestational age. Zhonghua Er Ke Za Zhi. 2015;53:97–103.
Wang DY, Ding WJ, Ding CC, Chen HT, Zhao WH, Sun B, et al. Higher peripheral cholesterol and a positive correlation with risk for large-for-gestational-age neonates in pre-pregnancy underweight women. Front Endocrinol (Lausanne). 2021;12:760934.
Farhat I, Moore L, Porgo TV, Assy C, Belcaid A, Berthelot S, et al. Interhospital variations in resource use intensity for in-hospital injury deaths: a retrospective multicenter cohort study. Ann Surg. 2022;275:e107–14.
Lenth RV. Least-squares means: the R package lsmeans. J Stat Softw. 2016;69:1–33.
Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. Sage Publications: London, 2002.
Beyerlein A, Schiessl B, Lack N, von Kries R. Optimal gestational weight gain ranges for the avoidance of adverse birth weight outcomes: a novel approach. Am J Clin Nutr. 2009;90:1552–8.
He Y, Tam CH-T, Yuen LY, Catalano PM, Ma RC-W, Tam WH. Optimal gestational weight gain for Chinese women-analysis from a longitudinal cohort with childhood follow-up. Lancet Reg Health West Pac. 2021;13:100190.
Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43:962–70.
LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group, Voerman E, Santos S, Inskip H, Amiano P, Barros H, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA 2019;321:1702.
Wu JN, Gu WR, Xiao XR, Zhang Y, Li XT, Yin CM. Gestational weight gain targets during the second and third trimesters of pregnancy for women with gestational diabetes mellitus in China. Eur J Clin Nutr. 2019;73:1155–63.
Nohr EA, Vaeth M, Baker JL, Sørensen TI, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008;87:1750–9.
Yee LM, Cheng YW, Inturrisi M, Caughey AB. Gestational weight loss and perinatal outcomes in overweight and obese women subsequent to diagnosis of gestational diabetes mellitus. Obes (Silver Spring). 2013;21:e770–4.
Gou BH, Guan HM, Bi YX, Ding BJ. Gestational diabetes: weight gain during pregnancy and its relationship to pregnancy outcomes. Chin Med J (Engl). 2019;132:154–160.
Kiefer MK, Adebayo A, Cleary E, Klebanoff M, Costantine MM, Landon MB, et al. Gestational weight gain and adverse maternal and neonatal outcomes for pregnancies complicated by pregestational and gestational diabetes. Am J Perinatol. 2022;39:691–8.
Chasan-Taber L, Silveira M, Waring ME, Pekow P, Braun B, Manson JE, et al. Gestational weight gain, body mass index, and risk of hypertensive disorders of pregnancy in a predominantly Puerto Rican population. Matern Child Health J. 2016;20:1804–13.
Allen VM, Joseph K, Murphy KE, Magee LA, Ohlsson A. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: a population-based study. BMC Pregnancy Childbirth. 2004;4:17.
Emond JA, Karagas MR, Baker ER, Gilbert-Diamond D. Better diet quality during pregnancy is associated with a reduced likelihood of an infant born small for gestational age: an analysis of the prospective New Hampshire birth cohort study. J Nutr. 2018;148:22–30.
Gete DG, Waller M, Mishra GD. Effects of maternal diets on preterm birth and low birth weight: a systematic review. Br J Nutr. 2020;123:446–61.
Hunt KJ, Schuller KL. The increasing prevalence of diabetes in pregnancy. Obstet Gynecol Clin North Am. 2007;34:173–99.
Wan CS, Abell S, Aroni R, Nankervis A, Boyle J, Teede H. Ethnic differences in prevalence, risk factors, and perinatal outcomes of gestational diabetes mellitus: a comparison between immigrant ethnic Chinese women and Australian-born Caucasian women in Australia. J Diabetes. 2019;11:809–17.
Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev. 2017;18:350–69.
Acknowledgements
The authors are grateful to all physicians, nurses, and other staff members from the University Hospital Advanced Age Pregnant Cohort. We are also indebted to all participants in the cohort.
Funding
This study was supported by grants from the National Key Research and Development Program of China (2016YFC1000401). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
JL, YZ and HL conceived the study; ZC and YZ wrote the manuscript; ZC performed the statistical analysis; YW, HL and JL contributed to the critical review of the manuscript; YW, YZ, HL, ZC, HY and JL participated in the data acquisition; and JL had full access to all the data in the study, and takes responsibility for the integrity and analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Institutional Review Board of Peking University Third Hospital (approval number: IRB00006761-2016145), and all participants provided informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41430_2022_1238_MOESM1_ESM.pdf
Predictive probability curves of SGA, LGA, and sum of SGA and LGA with respect to GWG in women of AMA (a) and younger women (b) with GDM
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, Z., Wei, Y., Li, H. et al. Estimated optimal gestational weight gain for pregnant women with gestational diabetes mellitus: a prospective cohort study in China. Eur J Clin Nutr 77, 356–362 (2023). https://doi.org/10.1038/s41430-022-01238-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-022-01238-8