Abstract
The aim of this systematic review and meta-analysis was to evaluate the effects of anthocyanins-interventions on oxidative stress, inflammation, and lipid profile in patients undergoing hemodialysis. This systematic review and meta-analysis were registered on the International Prospective Register of Systematic Reviews (PROSPERO CRD42020209742). The primary outcome was anthocyanins-rich intervention on OS parameters and secondary outcome was anthocyanins-rich intervention on inflammation and dyslipidemia. RevMan 5.4 software was used to analyze the effect size of anthocyanins-rich intervention on OS, inflammation and dyslipidemia. Meta-analysis effect size calculations incorporated random-effects model for both outcomes 1 and 2. Eight studies were included in the systematic review (trials enrolling 715 patients; 165 men and 195 women; age range between 30 and 79 years). Anthocyanin intervention in patients undergoing hemodialysis decrease the oxidant parameters (std. mean: −2.64, 95% CI: [−3.77, −1.50], P ≤ 0.0001, I2 = 97%). Specially by reduction of malondialdehyde products in favor of anthocyanins-rich intervention (std. mean: −14.58 µmol.L, 95% CI: [−26.20, −2.96], P ≤ 0.0001, I2 = 99%) and myeloperoxidase (std. mean: −1.28 ηg.mL, 95% CI: [−2.11, −0.45], P = 0.003, I2 = 77%) against placebo group. Decrease inflammatory parameters (std. mean: −0.57, 95% CI: [−0.98, −0.16], P = 0.007, I2 = 79%), increase HDL cholesterol levels (std. mean: 0.58 mg.dL, 95% CI: [0.23, 0.94], P = 0.001, I2 = 12%) against placebo group. Anthocyanins-rich intervention seems to reduce oxidative stress, inflammatory parameters and improve lipid profile by increasing HDL cholesterol levels in patients with chronic kidney disease undergoing hemodialysis.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is characterized by a glomerular filtration rate (GFR) leading to reduced and/or increased urinary albumin excretion and has been recognized as having a great impact on worldwide public health [1, 2]. Likewise, CKD presents abnormalities in the renal structure or in its function, which implicates in damage health [3]. The worldwide prevalence of CKD is estimated at 8–16% and appears to be higher in women (14.6%) when compared to men (12.8%) [2, 4]. The CKD prevalence may be associated mainly with the increased prevalence of diabetes mellitus, hypertension and aging [5, 6]. Worldwide, it is estimated that 1.2 million people died of CKD [1]. Therefore, is crucial to advance coordinated public health policy initiatives to attenuate this disease.
Renal replacement therapies should be instituted when the GFR is <15 mL/min/1.73 m2, such as hemodialysis (HD) and peritoneal dialysis [7]. By the year 2030, it is estimated that 5.439 million people will use some renal replacement therapy [8]. With this advance, there is an increase in health expenses that impact patients, the health system and society. It is estimated that annual costs per chronic kidney patient is around $ 40,000 dollars [2, 9, 10]. Although HD is considered a life-support strategy, this treatment interferes on patients’ quality of life [7]. The risk of mortality during HD is ten to thirty times higher than the general population when compared by age, ethnicity and sex [2, 11]. HD treatment is also associated with cardiovascular damage and depression of immune system due to uremic toxicity. The extreme imbalance of oxidative stress (OS) and chronic inflammation has led to atherosclerosis and high mortality in these patients [12]. As possible causes of this pessimistic clinical context, studies have demonstrated that HD procedure is associated with the generation of free radicals by activating white blood cells from the biocompatibility of dialyzers and dialysate, the loss of endogenous antioxidants and the retention of uremic toxins [13]. In this sense, many non-pharmacological strategies are necessary to attenuate the OS and chronic inflammation in these patients.
Improving the exogenous antioxidant capacity by food consumption or supplementation with bioactive compounds, such as vitamins B, C, D, and E, coenzyme Q10, L-carnitine, α-lipoic acid, curcumin, and omega 3 polyunsaturated fatty acids has emerged as promising strategy which has shown the potential adjunctive treatment to pharmaceutical treatment, considered safe and low cost [14, 15]. Among bioactive compounds, anthocyanins are well-known flavonols with low toxicity [16]. Anthocyanins are polyphenolic pigments that have two benzene rings, connected by a C ring of pyrone, are red-orange to blue-violet in fruits, flowers and leaves [17]. They are found in berries, cherries, grapes, raspberries, red grapes, red wine, strawberries, açai (Brazilian fruit) and fruits with dark peels [18]. These active compounds have a number of hydroxyl groups present in the glycosylated B ring structure and are associated with scavenging pathway free-radical elimination, cyclooxygenase pathway, mitogen-activated protein kinase pathway, and inflammatory cytokine signaling [19, 20]. Anthocyanins-rich intervention in vivo and in vitro models present promising effects in the placebo of cardiometabolic disease, increase of antioxidant capacity [21], decrease of inflammatory parameters [22] and reduced systolic and diastolic blood pressure [23]. In human, clinical trials investigating the effect of anthocyanins in patients undergoing HD has shown encouraging results, reduced risk of cardiovascular disease, beneficial effects on OS, inflammatory parameters, and blood pressure [24,25,26].
Indeed, meta-analyzes investigating the impact of anthocyanins on exercise-induced OS on reduction of systemic and vascular inflammatory parameters and glucose metabolism reported important findings regarding cardio-protective effect in healthy individuals [27,28,29]. However, to date, there is no systematic review and meta-analysis investigating the impact of anthocyanins-rich intervention on health outcomes in patients undergoing HD therapy. We hypothesized that anthocyanins-rich intervention would improve OS by increasing antioxidant capacity and decreasing inflammatory parameters. Therefore, the aim of this systematic review and meta-analysis was to determine and quantify the effects of the anthocyanins-rich intervention on OS, inflammatory, and lipid parameters in patients undergoing HD.
Materials/subjects and methods
Search approach
This meta-analysis was performed in accordance with Preferred Reporting Items for Systematic Review and Meta- Analyses (PRISMA) [30] and registered on International Prospective Register of Systematic Reviews (PROSPERO CRD42020209742). We searched for references on Clinical Trial Register, Cochrane Trial Register, PubMed, SPORT Discus, Web of Science, Scopus, SciELO, and Cumulative Index to Nursing and Allied Health from the earliest record up to August 2020. The search strategy combined the terms “Anthocyanins” OR “Blueberries” OR “Blackberries” OR “Raspberries” OR “Cranberries” OR “Red grapes” OR “Red juice” OR “Elderberries” OR “Açai” OR “Euterpe” OR “Pomegranate” OR “Red wine” OR “Black grape” OR “Berries” AND “OS” OR “Antioxidant Response” OR “Oxidants” OR “Antioxidants” OR “Free radicals” OR “Malondialdehyde” OR “Catalase” OR “Glutathione” OR “Superoxide dismutase” AND “Renal replacement therapy” OR “Dialysis” OR “HD Patients” AND “Randomized clinical trial” AND Clinical Trial” OR “Randomized Controlled Trial”. Reference lists from original and review articles were reviewed to identify additional relevant studies. Titles and abstracts of retrieved articles were evaluated by two reviewers (ICVSM and MGM) to assess their eligibility for meta-analysis. In case of disagreements, a third reviewer evaluated the article (CSP). The full text was consulted when the abstract did not provide sufficient information.
Eligibility criteria
Eligibility criteria were determined according to PICOS (Population, Intervention, Comparators, Outcome and Study design) clinical trials in patients undergoing HD comparing anthocyanins-rich intervention with placebo or unexposed competing controls and reporting OS parameters (oxidative and antioxidants measures) were examined. After exclusion of duplicate publications, the identified articles were included in the quantitative analysis if they matched the following inclusion criteria: (1) patients undergoing HD with engaged in anthocyanin-rich (fruits or juice) intervention in its predominantly composition or purified anthocyanins, (2) interventions that included a minimum of 2 weeks were eligible for the analysis. The records were excluded if they presented the following exclusion criteria: (1) studies involving children, pregnant or individuals presenting severe disease, (2) studies not presenting measurements of OS; (3) non-original studies, (4) studies presenting a high risk of bias (>70%).
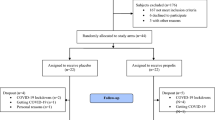
The search strategy considered two main outcomes: (1) anthocyanins-rich intervention on OS parameters (oxidative and antioxidants measures) - and (2) anthocyanins-rich intervention on inflammation and dyslipidemia in patients undergoing adjuvant therapy. Figure 1 describes the study selection process. Agreement between two authors (ICVSM and MGM) for the title/abstract screening was high (kappa = 0.932; <0.001).
Data extraction and study quality assessment
Two independent researchers (ICVSM and MGM) extracted study characteristics and rated the quality of included studies from published papers, using the ‘risk of bias’s assessment tool of the Cochrane Collaboration. The quality of following aspects was graded as high (‘+’), low (‘−’) or unclear (‘?’) quality [31]. Quality assessments of both reviewers were compared and disagreements in the scores were resolved by discussion. Study characteristics such as author/year of publication, sample size by anthocyanins-rich intervention and placebo, anthocyanins-rich intervention (ie, time of intervention, dosage, description of anthocyanins sources) (Table 1).
Study quantitative analysis
The meta-analysis procedures were conducted as described by Stroup et al. [32]. Pre and post anthocyanins-rich intervention data from OS parameters (oxidative parameters: malondialdehyde products, oxidized protein products, 8-hydroxy-2’-deoxyguanosine (8-OHdG), oxidized LDL, and antioxidant parameters: reduced glutathione (GSH), myeloperoxidase, paraoxonase (PON)-1, total antioxidant capacity), inflammatory parameters (C reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-a)), and lipid profile (triglycerides, total cholesterol, LDL and HDL cholesterol) were extracted from all studies included. In circumstances when standard deviations (SDs) were not available, these values were calculated using standard statistical methods assuming a correlation of 0.50 between the baseline and post-intervention scores within each subject [33]. Similarly, when studies reported standard error, the values were converted to SD. For studies with non-parametric data reporting median and range, the equations of Hozo et al. [34] were used to estimate mean and SD. Meta-analysis was conducted using Review Manager Software (RevMan software package version 5.4). RevMan was used to calculate the effect size of anthocyanins-rich intervention on OS in patients undergoing HD (outcome 1), or inflammation and dyslipidemia in patients undergoing HD (outcome 2).
Data from all included studies were used to calculate the standardized mean difference (Std. mean difference) and 95% confidence interval (CI) using a continuous random effects model for both outcomes 1 and 2. Weighted percentages were based on the sample sizes of respective studies. Statistical significance was assumed as p < 0.05 in a Z test analysis, to examine whether effect size was significantly different from zero. Study heterogeneity was evaluated using the I2 statistic and Cochrane’s Q. Values of I2 higher than 50 and 75% were considered moderate and high heterogeneity. For Cochrane’s Q, significant heterogeneity is considered to exist when the Q value exceeds the degrees of freedom (df) of the estimate [35]. Moreover, publication bias was tested visually using a funnel plot. Forest plots were generated to illustrate the study-level effect sizes along with a 95% CI [31, 35].
Meta-regression analysis
Meta-regression analysis was performed using the maximum likelihood method, the variation within-between studies as random effect and variable predictor as fixed effects [36]. Observations (effect size of oxidant parameters and days of anthocyanins intervention) were weighted by the inverse of the sampling variance [37]. An intercept-only model was created, estimating the weighted mean difference across all studies and exercise intervention groups [38]. Model parameter were estimated by the method of maximum likelihood [39]. Denominator degrees of freedom for statistical tests and CIs were calculated according to Berkey et al. (1995) [40]. Meta-regression was conducted using macros for Syntaxes (SPSS Inc., Chicago, IL, USA version 25 for Mac) and the bubble plot were performed on Microsoft Excel to illustrate the inverse variance.
Results
Studies’ characteristics
The selection process generated 616 articles and is documented in the PRISMA flow diagram (Fig. 1). Duplicate studies, reviews and meta-analyzes were excluded (n = 178). 438 studies were excluded for not presenting inclusion criteria and presenting inappropriate intervention. Three studies were identified in the reference list elsewhere meeting the eligibility criteria. A total of eight studies were included in the meta-analysis and presented low risk of bias (Fig. 2). The characteristics of the studies and the detailed description of the intervention protocol, use of anthocyanins and the results are presented in Table 1.
The total number of patients in the anthocyanins-rich intervention was 412 and 303 patients were assigned to placebo groups (165 men and 193 women; aged between 30 to 70 years). The length period of the anthocyanins-rich intervention varied from 14 days [41, 42] 4 to 10 weeks [26, 43] 6 months [44] and 1 year [45] The frequency of anthocyanins-rich intervention per week ranged from three times per week [26, 45] and between five and seven times a week [42,43,44, 46, 47]. Oxidants, antioxidants, inflammatory, and lipid parameters were used as our systematic review’s main results.
Oxidative parameters
Overall pooled analysis, there was a significant decrease on oxidative parameters favoring anthocyanins-rich intervention (std. mean: −2.64, 95% CI: [−3.77, −1.50], P ≤ 0.0001, I2 = 97%). The subgroup analysis showed significant reduction of malondialdehyde products in favor of anthocyanins-rich intervention (std. mean: −14.58 µmol.L, 95% CI: [−26.20, −2.96], P ≤ 0.0001, I2 = 99%) and myeloperoxidase (std. mean: −1.28 ηg.mL, 95% CI: [−2.11, −0.45], P = 0.003, I2 = 77%) against placebo group. On the other hand, there was a significant increase of 8-OHdG oxidative parameter (std. mean: 2.77 ηg.mL, 95% CI: [1.67, 3.88], P ≤ 0.0001, I2 = not applicable) favoring placebo group against anthocyanins-rich intervention. No significant difference was observed in oxLDL and AOPP oxidative parameters (Fig. 3). Regarding the potential moderating of ES for oxidant parameters, no meaningful moderation of length-term under anthocyanins-rich intervention during dialysis (adjusted R2 = 0.07, β = 0.009, P = 0.524) (Fig. 4).
Meta-analysis performed on the effects of anthocyanins on oxidants parameters (malondialdehyde products, oxidized protein products, 8-hydroxy-2’-deoxyguanosine (8-OHdG), oxidized LDL) in CKD patients undergoing HD. Calculation based on random effects model. Results are expressed as standardized mean difference (Std.MD) and 95% confidence intervals (95% CI). The diamonds represents magnitude of effect size, lines means 95% confidence interval and gray square represents weight for each study.
Antioxidant parameters
No significant improvement was found on antioxidant parameters (GSH, PON-1, total antioxidant capacity) in favor of anthocyanins-rich intervention in patients undergoing HD (std. mean: 1.22, 95% CI: [0.02, 2.43], I2 = 91%, P = 0.05) compared to placebo group (Fig. 5).
Meta-analysis performed on the effects of anthocyanins on antioxidants parameters (Reduced glutathione (GSH), myeloperoxidase, paraoxonase (PON)-1, total antioxidant capacity) in CKD patients undergoing HD. Calculation based on random effects model. Results are expressed as standardized mean difference (Std.MD) and 95% confidence intervals (95% CI). The diamonds represents magnitude of effect size, lines means 95% confidence interval and gray square represents weight for each study.
Inflammatory parameters
Overall pooled analysis, there was a significant decrease of inflammatory parameters in favor of anthocyanins-rich intervention (std. mean: −0.57, 95% CI: [−0.98, −0.16], P = 0.007, I2 = 79%). The subgroup analysis showed a significant reduction of IL-6 and TNF-α inflammatory parameters in favor anthocyanins-rich intervention (std. mean: −0.56 pg.mL), 95% CI: [−0.82, −0.30], P ≤ 0.0001, I2 = 37%), but not of CRP (std. mean: −0.67 mg.L), 95% CI: [−2.03, −0.63], P = 0.34, I2 = 91%) against placebo group in patients undergoing HD (Fig. 6).
Meta-analysis performed on the effects of anthocyanins on inflammatory parameters (C reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-a)) in CKD patients undergoing HD. Calculation based on random effects model. Results are expressed as standardized mean difference (Std.MD) and 95% confidence intervals (95% CI). The diamonds represents magnitude of effect size, lines means 95% confidence interval and gray square represents weight for each study.
Lipid parameters
Overall pooled analysis, there was no significant difference of lipid profile parameters (std. mean: 0.11, 95% CI: [−0.10, 0.33], P = 0.30, I2 = 41%). In contrast, the subgroup analysis showed a significant increase of HDL cholesterol parameter in favor anthocyanins-rich intervention (std. mean: 0.58 mg dL, 95% CI: [0.23, 0.94], P = 0.001, I2 = 12%) against placebo group, but not of triglycerides (std. mean: −0.17 mg.dL, 95% CI: [−0.57, 0.22], P = 0.39, I2 = 28%), total cholesterol (std. mean: −0.09 mg.dL, 95% CI: [−0.22, 0.41], P = 0.55, I2 = 0%), and LDL cholesterol (std. mean: 0.00 mg.dL, 95% CI: [−0.32, 0.31], P = 0.99, I2 = 0%) in patients undergoing HD (Table 2).
Discussion
This study summarized meta-analytical evidence supporting the effectiveness of anthocyanins-rich intervention attenuating the oxidative and inflammatory parameters in CKD patients undergoing HD therapy. Our initial hypothesis was partially accepted, anthocyanins attenuate OS by decreasing oxidant and inflammatory scenario, but not by increasing antioxidant capacity.
The inflammatory response is one of the important mechanisms for the development of CKD [48]. Increases on transcription nuclear factor- kB results in high expression of monocytes chemoattractant protein-1 which regulates the migration and infiltration of monocytes and macrophages, promoting local inflammation by an increased invasion of leukocytes and the enhanced production of reactive oxygen and nitrogen species (ROS/RNS), followed by the subsequent release pro-inflammatory cytokines, such as tumor necrosis factor-alpha and interleukin 1-beta (TNF-α and IL-1β), IL-6 among others [49]. The combination of kidney damage and exacerbate inflammation culminate to release of neutrophil gelatinase-associated lipocalin from neutrophils and kidney tubular epithelial cells [50].
Previous reviews and meta-analysis reported positive impact of anthocyanins in healthy individuals on cardiometabolic profile. It is known that the reduction in certain biomarkers such as lipid profile can prevent cardiovascular diseases [11], acting on vascular inflammation arising from free radicals that positively regulate cell adhesion molecules and chemokines [20, 51]. It is believed that the reduction in the protective capacity of HDL cholesterol against OS could be involved in the development of HD-induced atherosclerosis [52]. Our findings indicate a significant increase of serum HDL cholesterol levels in favors of anthocyanins-rich intervention compared with placebo group in patients undergoing HD; previous meta-analysis reported similar findings relationship between anthocyanin consumption. The previous meta-analysis reported similar findings between anthocyanin consumption [53,54,55]. This improvement may be due to anthocyanins’ action in inhibiting cholesteryl ester transfer protein [56] or by the change in paraoxonase-1 with improved HDL cholesterol efflux capacity [57].
Anthocyanin intake has been previously estimated for adult individuals (≥20 years) to be on average ~11.6 mg/day, in the range of 10.5 mg/day for men and 12.6 mg/day for women, which may significantly variate among different racial/ethnic groups populations in the United States [16], as well as for Chinese population are proposed 50 mg/day of anthocyanins consumption for prevention non-communicable diseases [58]. Despite the low toxicity, when it comes to food sources of anthocyanins such as berries, cherries, grapes, raspberries, red grapes, red wine, strawberries, açai (Brazilian fruit), and fruits with dark peels, they are associated with other minerals, like potassium, needs to monitor consumption and laboratory values to avoid the risk of hyperkalemia, common in renal patients [59, 60].
The mechanisms that may explain, at least in part, the modulation of OS-induced by anthocyanins is the possibly of act as scavengers in the excessive production of free radicals with increase of pathological progression [61]. In the uremia condition, there is a bidirectional and synergistic relationship between microinflammation—poorly adaptive, uncontrolled and persistent—and OS that contributes to cardiovascular risk [62,63,64]. HD-induced deleterious effect seems to be related to the duration of dialysis therapy, iron infusion, anemia, presence of central venous catheter and bioincompatible dialyzers [14]. Therefore, our findings suggest that decreasing anthocyanins-rich intervention-induced oxidant and inflammatory parameters, and increasing the HDL cholesterol may attenuate the uremia in patient undergoing HD.
Despite the previous findings in cardiovascular health biomarkers, no evidence of antioxidant capacity was found in this meta-analysis. Some factors need to be considered. Although there have been advances in the understanding of the effects of polyphenols on health, whether through the determination of potent polyphenols, the mixture of natural polyphenols and common biological activity, further understanding is still needed, especially when considering their low nano-micromolar concentration in circulation and tissues, conjugated metabolites and considerable inter-individual variability in the bioavailability [65,66,67]. Possibilities such as the use of studies with metabolomics and interventions with pure compounds are necessary [67].
While we are confident that we have retrieved the OS, inflammatory and lipid parameters studies, some expected limitations must be presented. High heterogeneity between-studies, especially due to the small number of studies included in the analysis (8 RCTs) and variability on number by group (anthocyanins and placebo) included in our meta-analysis. Noteworthy, absence in some clinical trials of the dose-dependent effect of anthocyanins’ dietary intake. The strong point in this meta-analysis is the pioneering on evaluating the clinical effects of anthocyanins-rich intervention in patients undergoing HD. We also encourage, further studies to investigate the effect of anthocyanins from different sources in both health and CKD patients.
Our findings indicate that anthocyanins-rich intervention promote decrease of OS by decreasing oxidative and inflammatory scenario, but not increasing antioxidant capacity. Furthermore, anthocyanins-rich intervention improves lipid profile by increasing HDL cholesterol in CKD patients undergoing HD.
Data availability
Registration and protocol; Template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review will be made available on request.
References
Bikbov B, Purcell CA, Levey AS, Smith M, Abobli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–33.
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260–72.
Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30.
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0158765.
Lv JC, Zhang LX. Prevalence and Disease Burden of Chronic Kidney Disease. Adv Exp Med Biol. 2019;1165:3–15.
Liu B, Lan H, Lv L. Renal Fibrosis: Mechanisms and Therapies. Advances in Experimental Medicine and Biology. Singapure: Springer; 1st ed. 2019.
Himmelfarb J, Ikizler TA. Hemodialysis. N Engl J Med. 2010;363:1833–45.
Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015;385:1975–82.
Wang V, Vilme H, Maciejewski ML, Boulware LE. The Economic Burden of Chronic Kidney Disease and End-Stage Renal Disease. Semin Nephrol. 2016;36:319–30.
Silva Junior GBD, Oliveira JGR, Oliveira MRB, Vieira LJES, Dias ER. Global costs attributed to chronic kidney disease: a systematic review. Rev Assoc Med Bras (1992). 2018;64:1108–16.
Neovius M, Jacobson SH, Eriksson JK, Elinder CG, Hylander B. Mortality in chronic kidney disease and renal replacement therapy: a population-based cohort study. BMJ Open. 2014;4:e004251.
Lowe F. Biomakers of oxidative stress. In: Laher I, editor. Systems of Biology of Free Radical and Antioxidants. 2. Berlin, Heidelberg:Springer; 2014. p. 65–87.
Liakopoulos V, Roumeliotis S, Gorny X, Dounousi E, Mertens PR. Oxidative stress in hemodialysis patients: a review of the literature. Oxid Med Cell Longev. 2017;2017:3081856.
Liakopoulos V, Roumeliotis S, Zarogiannis S, Eleftheriadis T, Mertens PR. Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin Dial. 2019;32:58–71.
Mafra D, Borges NA, Lindholm B, Shiels PG, Evenepoel P, Stenvinkel P. Food as medicine: targeting the uraemic phenotype in chronic kidney disease. Nat Rev Nephrol. 2020;3:153–171.
Sebastian RS, Enns CW, Goldman JD, Martin CL, Steinfeldt LC, Murayi T, et al. A new database facilitates characterization of flavonoid intake, sources, and positive associations with diet quality among US adults. J Nutr. 2015;145:1239–48.
Abdal Dayem A, Choi HY, Yang GM, Kim K, Saha SK, Cho SG. The Anti-Cancer Effect of Polyphenols against Breast Cancer and Cancer Stem Cells: Molecular Mechanisms. Nutrients. 2016;9:581.
Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–25.
Smeriglio A, Barreca D, Bellocco E, Trombetta D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother Res. 2016;30:1265–86.
Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61:1361779.
Ali T, Kim T, Rehman SU, Khan MS, Amin FU, Khan M, et al. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol Neurobiol. 2018;55:6076–93.
Aboonabi A, Singh I, Rose’ Meyer R. Cytoprotective effects of berry anthocyanins against induced oxidative stress and inflammation in primary human diabetic aortic endothelial cells. Chem Biol Interact. 2020;317:108940.
Asgary S, Sahebkar A, Afshani MR, Keshvari M, Haghjooyjavanmard S, Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother Res. 2014;28:193–9.
Spormann TM, Albert FW, Rath T, Dietrich H, Will F, Stockis JP, et al. Anthocyanin/polyphenolic-rich fruit juice reduces oxidative cell damage in an intervention study with patients on hemodialysis. Cancer Epidemiol Biomark Prev. 2008;17:3372–80.
Shema-Didi L, Kristal B, Ore L, Shapiro G, Geron R, Sela S. Pomegranate juice intake attenuates the increase in oxidative stress induced by intravenous iron during hemodialysis. Nutr Res. 2013;33:442–6.
Boldaji RB, Akhlaghi M, Sagheb MM, Esmaeilinezhad Z. Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: a randomized crossover trial. J Sci Food Agric. 2020;100:846–54.
Bloedon TK, Braithwaite RE, Carson IA, Klimis-Zacas D, Lehnhard RA. Impact of anthocyanin-rich whole fruit consumption on exercise-induced oxidative stress and inflammation: a systematic review and meta-analysis. Nutr Rev. 2019;9:630–45.
Fallah AA, Sarmast E, Fatehi P, Jafari T. Impact of dietary anthocyanins on systemic and vascular inflammation: systematic review and meta-analysis on randomised clinical trials. Food ChemToxicol. 2020;135:110922.
Fallah AA, Sarmast E, Jafari T. Effect of dietary anthocyanins on biomarkers of glycemic control and glucose metabolism: A systematic review and meta-analysis of randomized clinical trials. Food Res Int. 2020;137:109379.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 2021;372:n71.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 2011;343:d5928.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12.
Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769–73.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60.
Hox JL, Leeuw ED. Multilevel models for meta-analysis. Mahwah, NJ, US:Lawrence Erlbaum Associates Publishers; 2003. p. 90–111.
Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–25.
Jackson D, Riley RD. A refined method for multivariate meta-analysis and meta-regression. Stat Med. 2014;33:541–54.
Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693–708.
Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med. 1995;14:395–411.
Castilla P, Echarri R, Dávalos A, Cerrato F, Ortega H, Teruel JL, et al. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am J Clin Nutr. 2006;84:252–62.
Castilla P, Dávalos A, Teruel JL, Cerrato F, Fernández-Lucas M, Merino JL, et al. Comparative effects of dietary supplementation with red grape juice and vitamin E on production of superoxide by circulating neutrophil NADPH oxidase in hemodialysis patients. Am J Clin Nutr. 2008;87:1053–61.
Janiques AG, Leal Vde O, Stockler-Pinto MB, Moreira NX, Mafra D. Effects of grape powder supplementation on inflammatory and antioxidant markers in hemodialysis patients: a randomized double-blind study. J Bras Nefrol. 2014;36:496–501.
Wu PT, Fitschen PJ, Kistler BM, Jeong JH, Chung HR, Aviram M, et al. Effects of Pomegranate Extract Supplementation on Cardiovascular Risk Factors and Physical Function in Hemodialysis Patients. J Med Food. 2015;18:941–9.
Shema-Didi L, Sela S, Ore L, Shapiro G, Geron R, Moshe G, et al. One year of pomegranate juice intake decreases oxidative stress, inflammation, and incidence of infections in hemodialysis patients: A randomized placebo-controlled trial. Free Radic Biol Med. 2012;53:297–304.
Jafari T, Fallah AA, Bahrami M, Lorigooini Z. Effects of pomegranate peel extract and vitamin E on oxidative stress and antioxidative capacity of hemodialysis patients: A randomized controlled clinical trial. 2020a;72:104069.
Jafari T, Fallah AA, Reyhanian A, Sarmast E. Effects of pomegranate peel extract and vitamin E on the inflammatory status and endothelial function in hemodialysis patients: a randomized controlled clinical trial. 2020b;11:7987–7993.
Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol. 2009;24:1445–52.
Prasad GV. Metabolic syndrome and chronic kidney disease: current status and future directions. World J Nephrol. 2014;3:210–9.
van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–17.
Reis JF, Monteiro VV, de Souza Gomes R, do Carmo MM, da Costa GV, Ribera PC, et al. Action mechanism and cardiovascular effect of anthocyanins: a systematic review of animal and human studies. J Transl Med. 2016;14:315.
Morena M, Cristol JP, Canaud B. Why hemodialysis patients are in a prooxidant state? What could be done to correct the pro/antioxidant imbalance. Blood Purif. 2000;18:191–9.
Zhu Y, Miao Y, Meng Z, Zhong Y. Effects of Vaccinium Berries on Serum Lipids: A Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Altern Med. 2015;2015:790329.
Liu C, Sun J, Lu Y, Bo Y. Effects of anthocyanin on serum lipids in dyslipidemia patients: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0162089.
Shah K, Shah P. Effect of anthocyanin supplementations on lipid profile and inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Cholesterol 2018;2018:8450793.
Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, et al. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009;90:485–92.
Zhu Y, Huang X, Zhang Y, Wang Y, Liu Y, Sun R, et al. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J Clin Endocrinol Metab. 2014;99:561–9.
Clifford MN. Anthocyanins - nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1063–72.
Gilligan S, Raphael KL. Hyperkalemia and Hypokalemia in CKD: Prevalence, Risk Factors, and Clinical Outcomes. Adv Chronic Kidney Dis. 2017;24:315–8.
Brookes EM, Snider J, Hart GK, Robbins R, Power DA. Serum potassium in chronic kidney disease: prevalence, patient characteristics and clinical outcomes. Intern Med J. 2020;11:1906–1918.
Speer H, D'Cunha NM, Alexopoulos NI, McKune AJ, Naumovski N. Naumovski N. Anthocyanins and Human Health-A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants (Basel). 2020;5:366.
Libetta C, Sepe V, Esposito P, Galli F, Dal, Canton A. Oxidative stress and inflammation: implications in uremia and hemodialysis. Clin Biochem. 2011;44:1189–98.
Himmelfarb J, Hakim RM. Oxidative stress in uremia. Curr Opin Nephrol Hypertens. 2003;12:593–8.
Cobo G, Lindholm B, Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transpl. 2018;33:iii35–iii40.
Hollman PCH, Cassidy A, Comte B, Heinonen M, Richelle M, Richling E, et al. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J Nutr. 2011;141:989S–1009S.
Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, et al. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. Am J Clin Nutr. 2013;97:995–1003.
Hollman PCH. Unravelling of the health effects of polyphenols is a complex puzzle complicated by metabolism. Arch Biochem Biophys. 2014;1:100–5.
Funding
This study was supported by São Paulo Research Foundation (FAPESP). CSP received a Post Doctorate scholarship from the FAPESP (Process 2018/23402-0).
Author information
Authors and Affiliations
Contributions
ICVSM and CSP coordinated all the review steps and contributed to the interpretation and revised paper. MGM, JLMdN, DM, and AFS contributed to write and revise the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Martins, I.C.V.S., Maciel, M.G., do Nascimento, J.L.M. et al. Anthocyanins-rich interventions on oxidative stress, inflammation and lipid profile in patients undergoing hemodialysis: meta-analysis and meta-regression. Eur J Clin Nutr 77, 316–324 (2023). https://doi.org/10.1038/s41430-022-01175-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-022-01175-6