Abstract
The Epi-GEICAM study comprises 1017 invasive BC cases matched with controls of similar age (49 ± 9 years) and residence. Diet and OO consumption were collected through a validated food frequency questionnaire. 75% of women referred OO, common (refined) or virgin, as the main fat source. Using conditional logistic regression models, we compared different scenarios of type and frequency of OO consumption, using as reference those women not always using OO for the three culinary practices (seasoning, cooking, and frying) and adding <2 tablespoons (tbsps.) per day during the meal to bread, salad, or dishes. A substantial inverse association was observed in those women always using VOO for the three culinary practices and consuming ≥2 tbsps. of OO per day during meals (adjusted OR, 0.72; 95% CI: 0.51, 1.03; P = 0.07). Potential benefits from OO consumption, at least as regards the protection provided for BC, could be mostly conferred with VOO, and when its consumption is high.
Similar content being viewed by others
Breast cancer (BC) remains the most frequently diagnosed non-cutaneous malignant tumor in women in the majority of the countries worldwide abruptly disrupting the lives of millions of women [1]. Historically, a lower incidence of BC has been observed in Mediterranean countries [2] where, before the incursion of the fast-food culture, olive oil (OO) was the main culinary and unrestricted dressing fat both in the kitchen and at the table [2].
Reviewed results from observational studies provide supporting evidence for the association between Mediterranean diet (MeDiet), in which OO is the foremost source of fat, and decreased BC risk [3, 4]. Likewise, in the PREDIMED trial, which included the incidence of BC as a secondary outcome [5], the protective effect detected appeared to be mostly due to the very large consumption of virgin olive oil (VOO), which accounted for 22% of total caloric intake (≥50 g per day), among participants allocated to the MeDiet supplemented with VOO. Among 4152 women included in the trial, women allocated to the MeDiet supplemented with VOO group exhibited a 68% relatively lower risk of incident BC (hazard ratio [HR] = 0.32, 95% CI 0.13, 0.79) compared to the control group, where participants were recommended to follow a low-fat diet. Therefore, the PREDIMED trial provides first-level scientific evidence on BC protective properties of VOO within the context of the MeDiet [5]. Nevertheless, when based on observational studies, the level of certainty of the association between OO and BC is very low [6]. This may be, at least partly, because studies have not differentiated whether it was VOO or common OO (mostly based on refined oil).
Part of the salubrious health effects of OO may be due to its content in oleic acid, a monounsaturated fatty acid, which is found in all types of OO, but an important part is suggested to be attributable to the >200 minor components present in the VOO such as the phenolic compounds, but also tocopherols, phytosterols, carotenoids, luteolin, and triterpenic acids [7, 8]. VOO is obtained by cold pressing of ripe olives, retaining highly bioactive compounds of olives. However, most of these minor components are lost during refinery and consequently they are almost absent in common OO, which is sold as a mixture of refined OO (usually > 80%) and VOO [9].
In this context, we used data from the Epi-GEICAM study [10], which comprises 1017 confirmed invasive BC cases recruited between 2006 and 2011 in Spain and matched with healthy controls of similar age (49 ± 9 years) and residence. The global participation rate was 82%. BC cases and controls filled a comprehensive questionnaire which recorded demographic and anthropometric data, personal and family background, medical and occupational history, and lifestyle and dietary information. Type and frequency of OO consumption was comprehensively collected through a validated self-fulfilled semiquantitative food frequency questionnaire [11, 12]. Thus, participants answered how many tbsps. of OO, and of other vegetable oils (sunflower, corn, soy) they used to add at the table to salad, bread, and dishes. They also indicated what type of fat or oil they used for dressing, cooking, and frying, being able to select for each of these culinary practices among VOO (comprising virgin and extra virgin), common OO (mostly refined), sunflower, soy, corn, margarine, butter, and others.
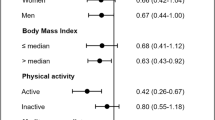
A total of 969 paired BC cases and controls (N = 1938) had complete data on OO consumption. 75% of women referred OO (either virgin or common) as the main fat source. The associations were evaluated using conditional logistic regression models adjusted for several potential confounders Table 1. Our assessment revealed that those women who added ≥ 2 tbsps. per day of OO (either virgin or common) during their meal to bread, salad, or their dish, presented a 12% lower risk of developing BC than those women who added <2 tbsps. per day (adjusted OR, 0.88; 95% CI: 0.70, 1.11; P = 0.29). When looking into the type of oils used for seasoning, cooking, and frying, those women using VOO for the three culinary practices had a non-significant 29% lower risk of BC (adjusted OR, 0.71; 95% CI: 0.40, 1.25; P = 0.24), when compared with those who used common OO for the three culinary practices. Finally, we compared different scenarios of OO consumption, using as reference those women not always using OO for the three culinary practices and adding <2 tbsps. per day during the meal to bread, salad, or dishes. Remarkably, a noteworthy inverse association was only observed in those women always using VOO for seasoning, cooking, and frying and moreover added ≥2 tablespoons of OO per day to bread, salad, or their dishes during meals, with a ~30% lower risk of BC (adjusted OR, 0.72; 95% CI: 0.51, 1.03; P = 0.07). This observed influence of olive oil on BC is independent of the other components of the Mediterranean diet.
Epidemiological evidence accumulated thus far show that OO may play a major role in explaining the associations of the MedDiet with a lower incidence of several chronic diseases such as cardiovascular disease, type 2 diabetes, neurodegenerative diseases, and cancer [8, 13].
Animal studies also suggest that high VOO diet may act as a negative modulator of the experimental mammary carcinogenesis, conferring to the tumors a more benign clinical behavior and a lower histopathological malignancy in comparison with high corn oil diets [14]. Hydrophilic phenols—including phenolic alcohols and acids, flavonoids, lignans and secoiridoids—are present in olive fruit as the most abundant VOO phenolic antioxidants, while they are not generally present in other oils and fats. They have also been attributed anti-inflammatory and anti-carcinogenic properties [15]. One of the VOO phenolic compound with major anti-inflammatory attributes is the oleocanthal, with an effect comparable even to that of non-steroidal anti-inflammatory drugs, like ibuprofen [16, 17]. Oleocanthal also has the potential to inhibit the growth of hormone-dependent BC and to improve the sensitivity to tamoxifen therapy in vitro and in vivo [18]. With regards hydroxytyrosol, the main phenolic compound present in VOO, it showed in vitro to act on both initiation and promotion/progression phases of BC carcinogenesis [19]. Oleacein, another phenolic compound found in VOO, showed to suppress in vitro the functional traits of BC cancer stem cells [20]. Nevertheless, observations in rodents often do not apply to human physiology and cannot be directly extrapolated to humans, since polyphenol metabolism differs. Likewise, data from in vivo studies report effects obtained with supra-physiological concentrations, where VOO polyphenols are extensively metabolized and reach the cells in minute amounts [21].
Based on our observations, benefits from OO consumption, at least as regards the protection provided for BC, could be only conferred with the virgin version, and when its consumption is high, at least ≥2 daily tbsps. While in the PREDIMED trial the protective effect (in both cardiovascular disease and BC) was detected with VOO consumptions ≥50 g per day (3 1/2 tbsps.) [5], unrestricted consumption should be avoided since the effects of very high consumption are not clearly established [22], while a high calorie intake increases breast cancer risk [23].
We want to call for epidemiological studies that fully consider type and amount of OO consumption to be able to properly characterize the OO potential for BC prevention and to quantify dose-response associations.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. https://doi.org/10.3322/caac.21660.
Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol Biomark Prev. 2000;9:869–73.
van den Brandt PA, Schulpen M. Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int J Cancer. 2017;140:2220–31. https://doi.org/10.1002/ijc.30654.
Dianatinasab M, Rezaian M, HaghighatNezad E, Bagheri-Hosseinabadi Z, Amanat S, Rezaeian S, et al. Dietary patterns and risk of invasive Ductal and Lobular Breast Carcinomas: a systematic review and meta-analysis. Clin Breast Cancer. 2020;20:e516–28. https://doi.org/10.1016/j.clbc.2020.03.007.
Toledo E, Salas-Salvado J, Donat-Vargas C, Buil-Cosiales P, Estruch R, Ros E, et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED Trial: a randomized clinical trial. JAMA Intern Med. 2015;175:1752–60. https://doi.org/10.1001/jamainternmed.2015.4838.
Sealy N, Hankinson SE, Houghton SC. Olive oil and risk of breast cancer: a systematic review and dose-response meta-analysis of observational studies. Br J Nutr. 2020:1–9. https://doi.org/10.1017/S0007114520003499.
Estruch R, Lamuela-Raventos RM, Ros E. The bitter taste of extra virgin olive oil for a sweet long life. J Am Coll Cardiol. 2020;75:1740–2. https://doi.org/10.1016/j.jacc.2020.02.043.
Gaforio JJ, Visioli F, Alarcon-de-la-Lastra C, Castaner O, Delgado-Rodriguez M, Fito M, et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019; 11. e-pub ahead of print 2019/09/05; https://doi.org/10.3390/nu11092039.
Yubero-Serrano EM, Lopez-Moreno J, Gomez-Delgado F, Lopez-Miranda J. Extra virgin olive oil: more than a healthy fat. Eur J Clin Nutr. 2019;72:8–17. https://doi.org/10.1038/s41430-018-0304-x.
Castello A, Pollan M, Buijsse B, Ruiz A, Casas AM, Baena-Canada JM, et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case-control EpiGEICAM study. Br J Cancer. 2014;111:1454–62. https://doi.org/10.1038/bjc.2014.434.
Vioque J, Navarrete-Munoz EM, Gimenez-Monzo D, Garcia-de-la-Hera M, Granado F, Young IS, et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J. 2013;12:26. https://doi.org/10.1186/1475-2891-12-26.
Martin-Moreno JM, Boyle P, Gorgojo L, Maisonneuve P, Fernandez-Rodriguez JC, Salvini S, et al. Development and validation of a food frequency questionnaire in Spain. Int J Epidemiol. 1993;22:512–9. https://doi.org/10.1093/ije/22.3.512.
Visioli F, Franco M, Toledo E, Luchsinger J, Willett W, Hu F, et al. Olive oil and prevention of chronic diseases: Summary of an International conference. Nutr Metab Cardiovasc Dis. 2018;28:649–56.
Solanas M, Hurtado A, Costa I, Moral R, Menéndez JA, Colomer R, et al. Effects of a high olive oil diet on the clinical behavior and histopathological features of rat DMBA-induced mammary tumors compared with a high corn oil diet. 2002; 21:745–53.
Servili M, Esposto S, Fabiani R, Urbani S, Taticchi A, Mariucci F, et al. Phenolic compounds in olive oil: antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology. 2009;17:76–84. https://doi.org/10.1007/s10787-008-8014-y.
Beauchamp GK, Keast RS, Morel D, Lin J, Pika J, Han Q, et al. Phytochemistry: ibuprofen-like activity in extra-virgin olive oil. Nature. 2005;437:45–46. https://doi.org/10.1038/437045a.
Harris RE, Beebe-Donk J, Alshafie GA. Reduction in the risk of human breast cancer by selective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2006;6:27. https://doi.org/10.1186/1471-2407-6-27.
Ayoub NM, Siddique AB, Ebrahim HY, Mohyeldin MM, El Sayed KA. The olive oil phenolic (-)-oleocanthal modulates estrogen receptor expression in luminal breast cancer in vitro and in vivo and synergizes with tamoxifen treatment. Eur J Pharm. 2017;810:100–11. https://doi.org/10.1016/j.ejphar.2017.06.019.
Rosignoli P, Fuccelli R, Sepporta MV, Fabiani R. In vitro chemo-preventive activities of hydroxytyrosol: the main phenolic compound present in extra-virgin olive oil. Food Funct. 2016;7:301–7. https://doi.org/10.1039/c5fo00932d.
Corominas-Faja B, Cuyas E, Lozano-Sanchez J, Cufi S, Verdura S, Fernandez-Arroyo S, et al. Extra-virgin olive oil contains a metabolo-epigenetic inhibitor of cancer stem cells. Carcinogenesis. 2018;39:601–13. https://doi.org/10.1093/carcin/bgy023.
Galmes S, Reynes B, Palou M, Palou-March A, Palou A. Absorption, distribution, metabolism, and excretion of the main olive tree phenols and polyphenols: a literature review. J Agric Food Chem. 2021;69:5281–96. https://doi.org/10.1021/acs.jafc.1c00737.
Tome-Carneiro J, Crespo MC, Lopez de Las Hazas MC, Visioli F, Davalos A. Olive oil consumption and its repercussions on lipid metabolism. Nutr Rev. 2020;78:952–68. https://doi.org/10.1093/nutrit/nuaa014.
Lope V, Martin M, Castello A, Ruiz A, Casas AM, Baena-Canada JM, et al. Overeating, caloric restriction and breast cancer risk by pathologic subtype: the EPIGEICAM study. Sci Rep. 2019;9:3904. https://doi.org/10.1038/s41598-019-39346-4.
Acknowledgements
We thank to all study participants.
Funding
This study was funded by the Fundación Científica Asociación Española Contra el Cancer (AECC) (Scientific Foundation of the Spanish Association against Cancer 2006 & 2016), Sociedad Española de Oncología Médica (SEOM) (Spanish Society of Medical Oncology), Community of Madrid (Scholarship “Contrato de atracción de talento” to Carolina Donat-Vargas), Fundación Cerveza y Salud 2005 (Beer and Health Foundation 2005) and Federación de Asociaciones de Mujeres con Cáncer de Mama (FECMA) (Spanish Federation of Associations of Women with Breast Cancer).
Author information
Authors and Affiliations
Contributions
CDV: formal analysis, writing - original draft, writing - review & editing. ÁGZ, BB, AC, JMBC, SA, PSR, AA, JÁGS, MR, MM, AJC, CJS, JIC, MGG, RAC, AL, SB: investigation, writing - review & editing. VL, NFLB, BPG: writing - review & editing. MM: conceptualization, investigation, writing - review & editing, supervision, project administration. MP: conceptualization, investigation, writing - review & editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
ÁGZ has received institutional grant from Pfizer; has participated in advisory board activities with Novartis, Palex, Pfizer and Astra Zeneca; has received travel grand from Roche, Pfizer and Novartis. MR has received honoraria from Novartis, Roche and Pfizer. MM has received honoraria from Roche/Genentech, Lilly, Pfizer, Novartis and Pierre-Fabre; has participated in consulting or advisory board activities with Roche/Genentech, Novartis, Pfizer, Lilly, Astra Zeneca and Taiho Pharmaceutical; has received speakers’ bureau from Lilly/ImClone, Roche/Genentech and Pierre Fabre; has contracted research fees from Roche, Novartis, and PUMA. All remaining authors have declared no conflicts of interest. Industry was not involved in the study hypothesis/design, execution, analysis, or interpretation.
Ethical approval and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committees, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The present study was approved by the Clinical Research Ethics Committees of the following institutions: Fundación Instituto Valenciano de Oncología; Hospital Universitario Virgen del Rocío de Sevilla; Hospital Universitario Puerta del Mar; Hospital Clínico San Carlos de Madrid; Hospital Clinic de Barcelona; Hospital Clínico Universitario de Valencia; Fundación Hospital Alcorcón; Complejo Hospitalario de Toledo; Hospital Mutua de Terrassa; Hospital Universitari de Bellvitge; Hospital General Universitario de Alicante; Hospital Virgen de los Lirios de Alcoy; Hospital Universitari de Girona Dr. Josep Trueta; Hospital Mutua de Terrassa; Hospital Universitari Arnau de Vilanova de Lleida; Fundacio d. Osona per a la Recerca i Educacio Sanitaries – FORES, and by the Regional Institutional Review Boards of Burgos and Soria, Aragon-CEICA, Galicia, Cantabria and Jaen. All participants signed an informed consent and patient information was anonymized and de-identified prior to analysis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Donat-Vargas, C., Guerrero-Zotano, Á., Lope, V. et al. Type does matter. Use VIRGIN olive oil as your preferred fat to reduce your risk of breast cancer: case-control EpiGEICAM study. Eur J Clin Nutr 76, 1343–1346 (2022). https://doi.org/10.1038/s41430-022-01101-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-022-01101-w
This article is cited by
-
Consumption of olive oil and risk of breast cancer in U.S. women: results from the Nurses’ Health Studies
British Journal of Cancer (2023)