Abstract
Background/Objectives
Routine use of vitamin D supplements has increased substantially in the United States. However, the safety and tolerability of long-term use of high-dose vitamin D are not known. We assessed the safety and tolerability of high-dose, daily vitamin D3 in the vitamin D and type 2 diabetes (D2d) study.
Subjects/Methods
In total, 2423 overweight/obese persons with prediabetes were randomized in a double-blind manner to either 4000 IU of vitamin D3 (the tolerable upper intake level for adults by the National Academy of Medicine) taken daily or matching placebo. All participants were included in this analysis. Incident adverse events (AE) were ascertained 4 times a year at in-person visits (twice a year) and interim remote encounters (twice a year) and were defined as untoward or unfavorable medical occurrences. Serious adverse events (SAE) included death, life-threatening events, and hospitalizations.
Results
A total of 8304 AEs occurred during 3 years of follow-up and were less frequent in the vitamin D group compared to placebo (Incidence Rate Ratio [IRR] = 0.94; 95% Confidence Interval (CI) 0.90, 0.98). The overall frequency of protocol-specified AEs of interest, which included nephrolithiasis, hypercalcemia, hypercalciuria, or low estimated glomerular filtration rate, was low and did not differ by group. There were no significant between-group differences in total SAEs (IRR = 0.96 (0.81, 1.14)).
Conclusion
Vitamin D3 supplementation at 4000 IU per day was safe and well tolerated among overweight/obese participants at high risk for diabetes who were appropriately monitored for safety. In this population, this dose of vitamin D3 did not increase risk of AEs or SAEs, including those previously associated with vitamin D such as hypercalcemia, hypercalciuria, or nephrolithiasis.
Clinical Trial Registration
ClinicalTrials.gov NCT01942694, prospectively registered September 16, 2013
Similar content being viewed by others
Introduction
There has been substantial interest in vitamin D and its potential role in prevention of a number of chronic diseases, including diabetes, cardiovascular disease, and cancer [1,2,3]. Recently, the focus of vitamin D research has turned to randomized controlled trials of vitamin D at doses higher than typically recommended compared to placebo in persons who were generally considered to be vitamin D sufficient by current guidelines [4,5,6,7,8,9]. However, there is insufficient evidence regarding the safety and tolerability of vitamin D supplementation at these higher doses [10, 11]. At the same time, routine use of vitamin D supplements, especially at doses higher than are typically recommended in guidelines, has increased substantially in the United States, despite insufficient data regarding potential safety issues or side effects with longer term use at these higher doses [12].
The Vitamin D and Type 2 Diabetes (D2d) study was a randomized clinical trial of vitamin D3 supplementation at a dose of 4000 IU per day compared to placebo among overweight/obese participants who were at high risk for type 2 diabetes [6]. In this pre-specified analysis, we examined the safety and tolerability of vitamin D3 supplementation in the D2d study, which tested the vitamin D dose that is considered the tolerable upper intake level (UL) for adults by the National Academy of Medicine [13].
Subjects and methods
Trial design overview
The D2d study was a randomized, double-blind, placebo-controlled clinical trial conducted to evaluate the safety and efficacy of oral vitamin D3 for diabetes prevention in adults at high risk for type 2 diabetes [5]. The study involved collaboration among 22 academic medical centers in the United States (d2dstudy.org/sites) [5]. The trial protocol is available at D2dstudy.org. A sponsor-appointed data and safety monitoring board approved the protocol and provided independent study monitoring. The institutional review board at each clinical site also approved the protocol, and all participants provided written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. The statistical team at the D2d coordinating center (Division of Endocrinology, Tufts Medical Center, Boston, USA) performed the statistical analysis and vouches for its accuracy.
Participants
Eligible participants met at least two of three glycemic criteria for prediabetes as defined by the 2010 American Diabetes Association (ADA) guidelines. Other inclusion criteria were age greater than or equal to 30 years (25 years for American Indians, Alaska Natives, Native Hawaiians, or other Pacific Islanders) and body mass index (BMI) of 24–42 kg/m2 (22.5–42 kg/m2 for Asian Americans) [14]. A low serum 25 hydroxyvitamin D (25[OH]D) concentration was not an inclusion criterion. Key exclusion criteria included use of diabetes or weight-loss medications or a history of hyperparathyroidism, nephrolithiasis, hypercalcemia, chronic kidney disease (defined as estimated glomerular filtration rate [eGFR] <50 mL/min/1.73 m2), calcium-to-creatinine ratio greater than 0.275 at baseline, or bariatric surgery. Persons were also excluded for use of supplements containing total doses of vitamin D higher than 1000 IU/day or total calcium higher than 600 mg/day. A complete list of exclusion criteria has been published and the recruitment process described previously [5, 6, 15].
Intervention and Procedures
Participants were randomized in a 1:1 allocation ratio to take once-daily, a soft-gel containing either 4000 IU of vitamin D3 (cholecalciferol) or matching placebo, with stratification by site, BMI (<30 or ≥30 kg/m2), and race (White or non-White). Participants and all study staff were blinded to treatment assignment. Participants were asked to limit the use of outside-of-study vitamin D to 1000 IU per day from all supplements. To optimize safety, participants were also asked to limit calcium supplements to 600 mg per day.
In-person follow-up visits occurred at month 3, month 6, and twice per year thereafter. Midway between the in-person visits starting after month 6, an interim contact (phone or email) took place. All visits and contacts were designed to promote retention, encourage adherence, and assess for diabetes diagnosis outside of the study, tolerability of study pills, occurrence of adverse events, and personal use of vitamin D supplements higher than allowed by study protocol. Participants were monitored for adverse events (AE) including those previously associated with vitamin D supplementation, and incident AEs were ascertained at visits and interim encounters 4 times a year in a similar manner in both groups. The protocol outlined in detail the safety parameters for which the study trial pills should be discontinued (e.g., nephrolithiasis, hypercalcemia, low eGFR, etc.) [6].
Vitamin D content of study trial pills was analyzed for each production lot at bottling for the vitamin D3 pills as well as for placebo pills to confirm they were free of vitamin D. Acceptable vitamin D3 content for the active vitamin D pill was pre-defined as 80–120% of the 4000 IU planned dosage.
Outcomes
The primary outcome of the D2d study was time to incident diabetes [14]. Participants who met the primary outcome of diabetes remained on the study pills and continued to be followed for safety and additional outcomes. The primary results have been previously published [6].
Adverse events were ascertained at each participant contact by study staff. At these encounters, each participant was asked if they had experienced any changes to their health or had sought medical care since last contact. If the participant responded affirmatively, study staff collected information on the health change or reason for and timing of medical care including diagnostic tests, diagnosis, and treatment. Study staff also reviewed with participants previously reported ongoing AEs to determine if the event had resolved.
An AE was defined as any untoward or unfavorable and unintended medical occurrence (including symptom, physical sign, laboratory finding, or disease) observed in or experienced by a participant, whether or not it was considered study related. A serious adverse event (SAE) was defined as any AE that resulted in death, a life-threatening event, a new inpatient hospitalization or prolongation of an existing hospitalization, a persistent or significant disability or incapacity, a congenital anomaly or birth defect, or any other significant hazard that, based upon appropriate medical judgment by the investigators, may have jeopardized the participant’s health and may have required medical or surgical intervention to prevent one of the outcomes listed in this definition. For all SAEs reported by the sites, medical records were collected and reviewed by the study’s Safety and Outcomes Subcommittee, composed of D2d investigators who were blinded to the participant’s assignment.
Key protocol-specified AEs of interest included hypercalcemia, hypercalciuria, low eGFR and nephrolithiasis. Follow-up serum calcium (assessed at each site’s local laboratory), the urine calcium-to-creatinine ratio (assessed at the central laboratory), and serum creatinine (assessed at each site’s local laboratory) to estimate GFR (calculated centrally) were measured at month 3, and annually thereafter. If serum calcium value (uncorrected for albumin concentration) was greater than the site’s clinical laboratory upper level of normal and less than or equal to the upper level of normal plus 1 mg/dL, participants were queried about calcium intake and supplements and medications (e.g., use of hydrochlorothiazide) and educated about the use of calcium supplements. Testing was repeated within 6 weeks. If the repeat serum calcium value was greater than the site’s clinical laboratory upper level of normal, the participant was confirmed to have met the outcome of hypercalcemia; study pills were stopped, and the participant was referred to their health care provider. If the first measurement of calcium was greater than the site’s clinical laboratory upper level of normal plus 1 mg/dL, no repeat testing was required and the participant was considered to have met the outcome of hypercalcemia; study pills were stopped, and the participant was referred to their health care provider. Regarding the adverse event of low eGFR, if eGFR value was greater than 30 and less than 40 mL/min/1.73m2, testing was repeated within 4 weeks. If repeat eGFR was equal to or less than 30 mL/min/1.73m2, the participant was confirmed to have met the outcome of low eGFR, study pills were stopped, and the participant was referred to their health care provider. If the first eGFR measurement was equal to or less than 30 mL/min/1.73m2, then no repeat testing was required, and the participant was considered to have met the outcome of low eGFR; study pills were stopped, and the participant was referred to their health care provider. Regarding the adverse event of hypercalciuria, if urine calcium-to-creatinine ratio was greater than 0.375, participants were queried about calcium intake and supplements, and testing was repeated within 4 weeks. If repeat urine calcium-to-creatinine ratio remained greater than 0.375, then the participant was considered to have met the outcome of hypercalciuria; study pills were stopped, and the participant was referred to their health care provider. The 0.375 cutoff was chosen because it represents the calcium-to-creatinine ratio in a random spot urine specimen that corresponds to a 24-hour urine calcium of 400 mg/gram, which is the upper reference range for men.
Participants were asked to contact site staff to report the occurrence of a kidney stone and were additionally specifically queried about kidney stones at each contact (phone or in-person visit). All reports of kidney stone were included in the nephrolithiasis outcome and participants reporting a kidney stone were instructed to discontinue study pills. If available, medical records related to nephrolithiasis were collected and then adjudicated by the study’s safety and outcomes subcommittee. For all of the above key protocol-specified AEs of interest where study pills were stopped per protocol, the pills were discontinued without unmasking participants or study staff, and participants continued in the study and completed all subsequent planned visits and measurements including collection of the primary outcome and safety assessment.
The D2d study did not specifically query the participants regarding falls using a validated questionnaire, but injuries and musculoskeletal events were self-reported by participants and were included in the overall assessment of safety.
Additional protocol-specified AEs of interest reported in this analysis were potentially related to the study pills and included polyuria, nausea, vomiting, poor appetite, metallic taste, hyperphosphatemia, anemia, weakness, fatigue, insomnia, and headache (all self-reported).
Participants could request to discontinue study pills at any point and for any reason. Participants who discontinued the study pills regardless of reason (AE or personal choice) were followed for the efficacy outcome per intent-to-treat principle.
Laboratory testing
Serum calcium and creatinine were analyzed locally at each site, and eGFR was calculated centrally using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation adjusted for race, as the formula was commonly applied when the study was conducted [16]. Other blood and urine specimens (including for calcium-to-creatinine ratio) were shipped to the central laboratory at baseline and during follow up. Serum total 25(OH)D, which includes total 25(OH)D3 and total 25(OH)D2, from stored frozen fasting serum samples from the baseline and annual visits, was measured by liquid chromatography–tandem mass spectrometry with calibrators that are traceable to the National Bureau of Standards and Technology and validated by quarterly proficiency testing program administered by the Vitamin D External Quality Assessment scheme (DEQAS, United Kingdom) [17, 18]. The D2d study did not measure free 25(OH)D3 or free 25(OH)D2 levels.
Statistical analyses
The sample size for the parent study was determined based on a target of 508 diabetes outcome events. The rationale has been previously published [5].
The frequency of AEs was evaluated in intention-to-treat analyses that compared groups defined by the randomization procedure and included all participants irrespective of adherence to assigned treatment or the protocol. All events were considered to occur independently, and no adjustment has been made for multiple events occurring in the same person. Incidence rates of AEs or differences in proportions of AEs were compared between the two groups. No adjustments were made for multiple comparisons.
The data analyses were generated using SAS software (Version 9.4 Copyright © 2019 SAS Institute Inc., Cary, NC, USA).
Results
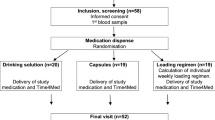
From October 2013 through February 2017, 7133 people were screened, and 2423 were randomly assigned to vitamin D3 (n = 1211) or placebo (n = 1212), forming the intention-to-treat population (Fig. 1) [6]. Of those randomized, 45% of participants were women; 33% were of a non-White race; and 9% were of Hispanic ethnicity (Table 1) [19]. Mean age was 60.0 years; body-mass index, 32.1 kg/m2; and HbA1c, 5.9% (48 mmol/mol). Mean baseline serum 25(OH)D concentration was 28.0 ng/mL (68.8 nmol/L) with 78.3% of participants having a concentration equal to or greater than 20 ng/mL (49.1 nmol/L). There were no statistically significant differences in baseline characteristics by treatment assignment.
2423 participants were randomized in D2d. 1211 were assigned to the vitamin D group and 1212 were assigned to the placebo group. Reasons for lost data or withdrawal from the study are presented as well as reasons for discontinuation of study pills by treatment assignment. 1201 participants in the vitamin D group completed at least one follow-up encounter and 1199 in the placebo group completed at least one follow-up encounter. 1123 participants in the vitamin D group died or completed last follow-up encounter and 1119 in the placebo group died or completed last follow-up encounter. 1211 in the vitamin D group and 1212 in the placebo group are included in these analyses.
The last study encounter was in December 2018 and the trial was stopped when the number of prespecified diabetes events had occurred per protocol. The overall median follow-up was 3.0 years (vitamin D3 3.0 [interquartile range, 2.0–3.6] years; placebo 2.9 [interquartile range, 2.0–3.5] years) and 99.1% of the cohort (1201 vitamin D3 and 1199 placebo group) contributed follow-up data.
The overall frequency of protocol-specified AEs of interest was low, with no significant between-group differences in the incidence of the first occurrence of the following protocol-specified adverse events of interest: hypercalcemia, hypercalciuria, hyperphosphatemia, low eGFR, metallic taste, fatigue / weakness, insomnia, polyuria, or nephrolithiasis (Table 2). There were 36 cases of participants with new-onset hypercalcemia on initial testing; on repeat testing, only 10 cases were confirmed, 6 in the vitamin D3 and 4 in the placebo group (incidence rate ratio [IRR] for vitamin D3 vs. placebo = 1.49; 95% CI 0.42, 5.27). There were 21 participants with new-onset hypercalciuria on initial testing; on repeat testing, only 2 cases were confirmed, 1 in each group (IRR for vitamin D3 vs. placebo = 0.99; 95% CI 0.06, 15.86). There were 3 cases of confirmed low eGFR, 1 in the vitamin D3 group and 2 in the placebo group (IRR for vitamin D3 vs. placebo = 0.50; 95% CI 0.04, 5.47). There were 52 self-reported cases of nephrolithiasis, 28 in the vitamin D3 and 24 in the placebo group (IRR for vitamin D3 vs. placebo = 1.16; 95% CI 0.67, 2.00) (Table 3). While the number of adverse events related to nausea/vomiting or poor appetite was low (n = 29), there were more cases reported among persons taking vitamin D3 compared to placebo, 20 vs 9 respectively (IRR for vitamin D3 vs. placebo = 2.20; 95% CI 1.00, 4.84). Supplementary Table 1 provides additional information on the total frequency of protocol-specified AEs of interest among persons who were taking their study pills at the time of the event.
A total of 8304 AEs occurred during follow-up. The incidence rate of total AEs was lower in the vitamin D3 group (4039; 116.1 events per 100 person-years) compared to the placebo group (4265; 123.6 events per 100 person years) (IRR = 0.94; 95% CI 0.90, 0.98) (Table 3). A total of 529 SAEs occurred during follow-up. The incidence rate of SAEs was not different between the vitamin D3 (260 events; 7.47 per 100 person-years) and placebo groups (269; 7.80 per 100 person-years) (IRR = 0.96; 95% CI 0.81, 1.14). The majority of SAEs were for hospitalization and there was no statistically significant difference among the treatment groups (IRR = 0.94; 95% CI 0.79, 1.12) (Table 3). Supplementary Tables 2, 3, and 4 provide additional data for total AEs and SAEs by treatment group using an end organ classification. The vitamin D3 group had fewer AEs and SAEs for injury and musculoskeletal events.
Adherence to the intervention was high (84.1% of prescribed pills were taken) and a similar proportion of participants in the vitamin D3 group (12.1%) and placebo group (10.3%) stopped trial pills (difference, 1.8 percentage points; 95% CI, −0.7, 4.3). There was no significant difference between the proportions of participants who stopped study pills for any reason, including due to AEs or due to participant choice (17.5% in the vitamin D group vs. 16.0% in the placebo group). There was no significant difference between the proportions of participants who stopped study pills due to an AE: overall, 58 (4.8%) participants in the vitamin D group stopped trial pills due to an AE, including abnormal safety labs, compared to 46 (3.8%) in the placebo group (difference in proportions for vitamin D vs. placebo, 0.9% [95% CI, −0.6, 2.6%]) (Table 4).
Discussion
In this multi‐center, randomized, double-blind, placebo‐controlled trial among overweight/obese persons at high risk of type 2 diabetes not selected for vitamin D insufficiency and who were screened and routinely monitored for safety, compared to placebo, oral vitamin D3 supplementation at a dose of 4000 IU per day (considered the UL for adults by the National Academy of Medicine) [13] was well-tolerated and did not result in an increased risk of AEs or SAEs, including side effects typically linked to vitamin D, such as hypercalcemia, nephrolithiasis, or hypercalciuria. This finding of no increased risk of AE or SAE in the vitamin D3 group is reassuring given that the majority of D2d participants began the trial with concentrations of serum 25(OH)D considered sufficient for healthy adults [6].
These data from the D2d study suggest that in similar populations of people who are overweight/obese and with prediabetes, the UL for safety and tolerability of vitamin D3 may be higher than previously established. The dose of vitamin D3 of 4000 IU per day used in the D2d study is the National Academy of Medicine recommended UL for persons over 8 years of age [13], and D2d participants were allowed to take up to 1000 IU of vitamin D3 on their own, for a maximum total dose of vitamin D of 5000 IU daily from supplements. The UL was established by the National Academy of Medicine in 2011 based on a synthesis of data indicating that a dose of 4000 IU was unlikely to cause hypercalcemia [20]. The National Academy of Medicine also chose a UL dose that would maintain a serum 25(OH)D concentration lower than 50–75 ng/mL (125–150 nmol/L), a concentration that was previously thought to be associated with adverse outcomes. However, the benefit-risk ratio may be different in populations that vary by BMI, skin complexion, or when a trial attempts to achieve higher 25(OH)D concentrations. Future trials are warranted to test the efficacy-safety ratio of higher doses of vitamin D supplementation that aim to achieve higher 25(OH)D levels in specific populations at-risk for specific conditions, such as diabetes, osteoporosis, cancer etc.
The dose of 4000 IU daily of vitamin D3 in D2d is a higher dose than administered in other recently completed clinical trials. For example, Lappe et al tested a 2000 IU daily dose of vitamin D3 and administered calcium supplements in addition to the vitamin D3 as part of the intervention arms in postmenopausal women [21, 22]; and the VITAL trial of adult men aged 50 or older and women aged 55 or older tested a dose of vitamin D3 2000 IU daily without calcium [7]. Other trials include the New Zealand ViDA trial which tested a dose of vitamin D3 100,000 IU taken monthly [23, 24]. Similar to the D2d study, these three trials have also reported few adverse events and no increased risk of hypercalcemia with vitamin D supplementation [7, 22, 23]. In contrast to the D2d study, only the Women’s Health Initiative Calcium / Vitamin D trial, with a much larger sample size and a longer follow-up period, reported an 17% increased risk of nephrolithiasis with combined daily 400 IU of vitamin D3 and 1000 mg of calcium compared to placebo (hazard ratio, 1.17; 95% CI, 1.02–1.34) [21].
There are trials with vitamin D supplementation that have reported other adverse events that were not assessed in the D2d study. A Canadian study found that, among healthy older adults (ages 55 to 70 years), vitamin D3 for 3 years at 4000 IU or 10,000 IU per day compared with 400 IU per day, resulted in statistically significant lower radial bone mineral density (BMD) as measured by high resolution peripheral quantitative computed tomography, but there were no significant differences in bone strength [25]. The D2d study did not assess BMD and thus cannot contribute information about the effect of the 4000 IU per day on BMD and bone strength in overweight/obese people with prediabetes. A trial conducted in Switzerland tested vitamin D3 at 60,000 IU per month vs. 24,000 IU per month and reported increased risk of falls with the higher dose [26]. The D2d study did not specifically query the participants regarding falls using a validated questionnaire; however the vitamin D3 had fewer AEs and SAEs for injury or musculoskeletal events than the placebo group (Supplementary Tables 2 and 4).
D2d is the first trial to assess the safety of vitamin D3 given at the tolerable upper intake level for adults by the National Academy of Medicine in a population of US adults with overweight/obesity and prediabetes. The D2d study has several additional strengths, including a large diverse group of participants who were at low risk for safety concerns related to vitamin D but at high risk for diabetes. The vitamin D3 dose of 4000 IU per day was selected to balance safety and efficacy and resulted in, on average, large differences in serum 25(OH)D concentrations between the vitamin D3 and placebo groups (54.3 ng/mL vs. 28.8 ng/mL, respectively, at month 24) [6]. Use of a placebo to blind investigators, staff, and participants to treatment assignment minimized ascertainment bias of adverse events, and careful attention to protocol fidelity resulted in a rigorously conducted clinical trial. Adverse events were collected frequently and in a similar way in both groups to reduce ascertainment bias. All serious adverse events and cases of nephrolithiasis were adjudicated by study investigators blinded to treatment assignment.
There are several considerations to put interpretation of our findings in context. As in all vitamin D trials, participants were excluded if they had a condition (e.g., high baseline serum calcium, etc.) that would have increased their risk for vitamin D-associated adverse events, and this may have reduced the occurrence of AEs compared to the general population taking vitamin D. The median time of follow-up in the D2d study was three years, and our findings may not extrapolate to longer term use of 4000 IU per day of vitamin D3. Thus, the risk of vitamin D associated AEs and SAEs may be greater among persons who are at higher risk, who take the supplement for longer periods of time, or who have been less carefully screened and monitored. Finally, the D2d study did not assess whether participants had a CYP24A1 mutation or other mutations in the vitamin D pathway, so we are unable to provide pharmacogenomic information.
Conclusion
High-dose daily vitamin D3 supplementation at a dose of 4000 IU daily (considered the tolerable upper intake level for adults by the National Academy of Medicine) was safe and well tolerated among overweight/obese participants with prediabetes who were screened for risk of AEs and monitored for safety; and use of this supplement did not increase risk of AEs or SAEs, including side effects that have been previously associated with vitamin D.
Data availability
Datasets generated and analyzed during the current study and the associated data dictionary and code are not publicly available. Requests for datasets analyzed and code utilized in the current study can be made after acceptance for publication by bona fide researchers by submitting a request to the D2d Publications Committee. Individual participant data will be shared in a de-identified/anonymized format using a specialized SAS data platform. Protocol synopsis, contact details, publications, and the process for collaboration and data requests can be found on the website (d2dstudy.org).
Change history
13 April 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41430-022-01130-5
References
Mondul AM, Weinstein SJ, Layne TM, Albanes D. Vitamin D and cancer risk and mortality: state of the science, gaps, and challenges. Epidemiol Rev. 2017;39:28–48.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 dietary reference intakes for calcium and vitamin D: what dietetics practitioners need to know. J Am Diet Assoc. 2011;111:524–7.
Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29.
Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–71.
Pittas AG, Dawson-Hughes B, Sheehan PR, Rosen CJ, Ware JH, Knowler WC, et al. Rationale and design of the vitamin D and type 2 diabetes (D2d) study: a diabetes prevention trial. Diabetes Care. 2014;37:3227–34.
Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520–30.
Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44.
Dawson-Hughes B, Staten MA, Knowler WC, Nelson J, Vickery EM, LeBlanc ES, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the vitamin D and type 2 diabetes (D2d) study. Diabetes Care. 2020;43:2916–22.
Chandler PD, Chen WY, Ajala ON, Hazra A, Cook N, Bubes V, et al. Effect of vitamin D3 supplements on development of advanced cancer: a secondary analysis of the VITAL randomized clinical trial. JAMA Netw Open. 2020;3:e2025850.
Moyer VA, Force USPST. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive services Task Force recommendation statement. Ann Intern Med. 2014;160:558–64.
Scragg R. Emerging Evidence of Thresholds for Beneficial Effects from Vitamin D Supplementation. Nutrients. 2018;10:561. https://doi.org/10.3390/nu10050561.
Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999–2012. JAMA. 2016;316:1464–74.
Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011;14:938–9.
American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care. 2010;33(Suppl 1):S11–61. https://doi.org/10.2337/dc10-S011.
Aroda VR, Sheehan PR, Vickery EM, Staten MA, LeBlanc ES, Phillips LS, Brodsky IG, Chadha C, Chatterjee R, Ouellette MG, Desouza C, Pittas AG; D2d Research Group. Establishing an electronic health record-supported approach for outreach to and recruitment of persons at high risk of type 2 diabetes in clinical trials: The vitamin D and type 2 diabetes (D2d) study experience. Clin Trials. 2019;16:306–315. https://doi.org/10.1177/1740774519839062.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Bedner M, Lippa KA, Tai SS. An assessment of 25-hydroxyvitamin D measurements in comparability studies conducted by the Vitamin D Metabolites Quality Assurance Program. Clin Chim Acta. 2013;426:6–11.
DEQAS (Vitamin D External Quality Assessment Scheme). [August 30, 2020]. Available from: http://www.deqas.org.
LeBlanc ES, Pratley RE, Dawson-Hughes B, Staten MA, Sheehan PR, Lewis MR et al. Baseline characteristics of the vitamin D and type 2 diabetes (D2d) study: a contemporary prediabetes cohort that will inform diabetes prevention efforts. Diabetes Care 2018.
Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Washington (DC): National Academies Press (US); 2011.
Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–83.
Lappe J, Watson P, Travers-Gustafson D, Recker R, Garland C, Gorham E, et al. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. 2017;317:1234–43.
Malihi Z, Lawes CMM, Wu Z, Huang Y, Waayer D, Toop L, et al. Monthly high-dose vitamin D supplementation does not increase kidney stone risk or serum calcium: results from a randomized controlled trial. Am J Clin Nutr. 2019;109:1578–87.
Neale RE, Armstrong BK, Baxter C, Duarte Romero B, Ebeling P, English DR, et al. The D-Health Trial: A randomized trial of vitamin D for prevention of mortality and cancer. Contemp Clin Trials. 2016;48:83–90.
Burt LA, Billington EO, Rose MS, Raymond DA, Hanley DA, Boyd SK. Effect of high-dose vitamin D supplementation on volumetric bone density and bone strength: a randomized clinical trial. JAMA. 2019;322:736–45.
Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176:175–83.
Acknowledgements
We thank the D2d investigators, staff, and trial participants for their dedication and commitment to the trial. Dr. Pittas is supported, in part, by generous donations to the Tupper Research Fund at Tufts Medical Center.
Funding
The planning phase of the D2d trial was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) through a multicenter clinical study implementation planning grant to Tufts Medical Center in Boston (U34DK091958; principal investigator, AGP). Planning was also supported, in part, by the Intramural Research Program of the NIDDK. The conduct of the trial was supported primarily by the NIDDK and the Office of Dietary Supplements of the National Institutes of Health through the multicenter clinical study cooperative agreement (U01DK098245; principal investigator, AGP) to Tufts Medical Center, where the D2d Coordinating Center is based. The U01 grant mechanism establishes the NIDDK project scientist (Dr. Staten) as a member of the D2d Research Group. The trial also received secondary funding from the American Diabetes Association to Tufts Medical Center (1-14-D2d-01; principal investigator, Dr. Pittas). Neither the funders nor the author’s institutions had any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the National Institutes of Health.
Author information
Authors and Affiliations
Consortia
Contributions
All authors (KCJ, AP, KLM, ALP, LSP, EMV, JN, PRS, DR, SM, RC) are responsible for the design and final content of the manuscript. JN performed the statistical analysis. KCJ and RC are responsible for drafting the manuscript and AP, KLM, ALP, LSP, EMV, JN, PRS, DR, SM are responsible for review and substantive revisions of the manuscript. All authors (KCJ, AP, KLM, ALP, LSP, EMV, JN, PRS, DR, SM, RC) have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The following authors report no conflicts of interest Karen Johnson, Anastassios Pittas, Karen Margolis, Anne Peters, Ellen Vickery, Jason Nelson, Patricia Sheehan, David Reboussin, Saul Malozowski, Ranee Chatterjee Lawrence Phillips reports the following: Grant Support: Supported in part by VA awards CSP #2008, I01 CX001899, I01 CX001737, and HSR&D IIR 07–138, NIH awards R21 DK099716, R18 DK066204, R03 AI133172, R21 AI156161, U01 DK091958, U01 DK098246, UL1 TR002378, and a Cystic Fibrosis Foundation award PHILLI12A0. Disclosure Statement: Dr. Phillips declares that there is no duality of interest associated with this manuscript. With regard to potential conflicts of interest, Dr. Phillips has served on Scientific Advisory Boards for Boehringer Ingelheim and Janssen, and has or had research support from Merck, Pfizer, Eli Lilly, Novo Nordisk, Sanofi, PhaseBio, Roche, Abbvie, Vascular Pharmaceuticals, Janssen, Glaxo SmithKline, and the Cystic Fibrosis Foundation. Dr. Phillips is also a cofounder and Officer and Board member and stockholder of a company, Diasyst, Inc., which markets software aimed to help improve diabetes management. Anne Peters reports the following: Dr. Peters has served on scientific advisory boards for: Abbott Diabetes Care, Biorad, Eli Lilly and Company, MannKind, Medscape, Merck, Novo Nordisk, Zealand Research Support: Dexcom, vTv Therapeutics, Abbott Diabetes Care Stock Options: Omada Health, Teladoc
Ethical approval
A sponsor-appointed data and safety monitoring board approved the protocol and provided independent study monitoring. The institutional review board at each clinical site also approved the protocol, and all participants provided written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Johnson, K.C., Pittas, A.G., Margolis, K.L. et al. Safety and tolerability of high-dose daily vitamin D3 supplementation in the vitamin D and type 2 diabetes (D2d) study—a randomized trial in persons with prediabetes. Eur J Clin Nutr 76, 1117–1124 (2022). https://doi.org/10.1038/s41430-022-01068-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-022-01068-8