Abstract
Background/objectives
Neuroendocrine neoplasms (NEN) may predispose patients to malnutrition. CT-defined sarcopenia and myosteatosis are common in other tumour types and recognized adverse prognostic factors. However, the prevalence and prognostic impact of sarcopenia and myosteatosis remain undetermined in NEN patients to date.
Methods
A retrospective study of NEN patients treated with peptide receptor radionuclide therapy (PRRT) at a tertiary institution from 2012 to 2017. Patients with PET/CT imaging at baseline and follow-up were included. The L3 slice of the co-localizing CT was analysed using the Alberta Protocol. Skeletal muscle cross-sectional area and muscle attenuation were measured and compared with pre-defined cut-offs. The primary endpoint was the prevalence of sarcopenia and myosteatosis according to previously published cut-offs.
Results
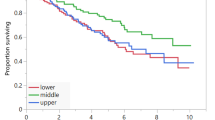
Fourty-nine patients (median age 64 (range 26–80) years) were included. The most common primary sites of tumour were the small bowel (51%) and pancreas (26%). Baseline sarcopenia was prevalent in 67% of patients and myosteatosis in 71%. Forty-five percent of patients gained weight over the course of PRRT. The presence of baseline sarcopenia was not associated with progression-free survival (20.8 mo vs. 20.7 mo, HR 0.86, p = 0.70) nor overall survival. Similarly, baseline myosteatosis (PFS 19.5 mo vs. 20.8 mo, HR 0.77, p = 0.47) was not significantly associated with survival outcomes. The mean (SD) age of those with myosteatosis was 60.8 ± 11.6 years compared to 49.7 ± 12.7 years for those without (p = 0.003).
Conclusions
Body composition analysis is feasible using routinely acquired PET/CT data for patients with NEN. CT-defined sarcopenia and myosteatosis are prevalent in NEN patients, although myosteatosis is more common with increasing age. These findings were not associated with worsened overall or progression-free survival in the current study.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42.
Halperin DM, Shen C, Dasari A, Xu Y, Chu Y, Zhou S, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol. 2017;18:525–34.
Beaumont JL, Cella D, Phan AT, Choi S, Liu Z, Yao JC. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas. 2012;41:461–6.
Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67.
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol J Am Soc Clin Oncol. 2013;31:1539–47.
Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res J Am Assoc Cancer Res. 2009;15:2920–6.
Qureshi SA, Burch N, Druce M, Hattersley JG, Khan S, Gopalakrishnan K, et al. Screening for malnutrition in patients with gastro-entero-pancreatic neuroendocrine tumours: a cross-sectional study. BMJ Open. 2016;6:e010765.
Maasberg S, Knappe-Drzikova B, Vonderbeck D, Jann H, Weylandt KH, Grieser C, et al. Malnutrition predicts clinical outcome in patients with neuroendocrine neoplasia. Neuroendocrinology. 2017;104:11–25.
Laing E, Kiss N, Michael M, Krishnasamy M. Nutritional complications and the management of patients with gastroenteropancreatic neuroendocrine tumours. Neuroendocrinology. 2019. https://www.karger.com/Article/FullText/503634.
Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. Clin Nutr Edinb Scotl. 2019;38:1–9.
Isenring E, Zabel R, Bannister M, Brown T, Findlay M, Kiss N, et al. Updated evidence-based practice guidelines for the nutritional management of patients receiving radiation therapy and/or chemotherapy: 2012 Radiation therapy and/or chemotherapy nutrition guidelines. Nutr Diet. 2013;70:312–24.
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48.
Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol J Eur Soc Med Oncol. 2018;29 Suppl 2:ii1–9.
Kazemi-Bajestani SMR, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol. 2016;54:2–10.
Martin L, Gioulbasanis I, Senesse P, Baracos VE. Cancer-associated malnutrition and CT-defined sarcopenia and myosteatosis are endemic in overweight and obese patients. J Parenter Enteral Nutr. 2020;44:227–38.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31.
Kiss N, Loeliger J, Findlay M, Isenring E, Baguley BJ, Boltong A, et al. Clinical Oncology Society of Australia: position statement on cancer‐related malnutrition and sarcopenia. Nutr Diet. 2020;77:416–25.
Stretch C, Aubin J-M, Mickiewicz B, Leugner D, Al-Manasra T, Tobola E, et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PloS ONE. 2018;13:e0196235.
Zamboni M, Gattazzo S, Rossi AP. Myosteatosis: a relevant, yet poorly explored element of sarcopenia. Eur Geriatr Med. 2019;10:5–6.
Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol A Biol Sci Med Sci. 2019;74:1671–8.
Pevny S, Maasberg S, Karber M, Knappe-Drzikova B, Weylandt KH, Pavel ME, et al. Systemic anti-cancer therapies in neuroendocrine tumor patients impair nutritional status. Neuroendocrinology. 106:Abst J18.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177 Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35.
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge M-P, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 1985. 2004;97:2333–8.
Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35.
Bosman FT, World Health Organization, International Agency for Research on Cancer, editors. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer; 2010. p. 417 World Health Organization classification of tumours.
Lloyd RV, Osamura RY, Klöppel G, Rosai J, editors. WHO classification of tumours of endocrine organs. 4. Auflage. Lyon: International Agency for Research on Cancer; 2017. p. 355.
Miljkovic I, Kuipers AL, Cvejkus R, Bunker CH, Patrick AL, Gordon CL, et al. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obesity. 2016;24:476–82.
Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–71.
Dunne RF, Roussel B, Culakova E, Pandya C, Fleming FJ, Hensley B, et al. Characterizing cancer cachexia in the geriatric oncology population. J Geriatr Oncol. 2019;10:415–9.
Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X, et al. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis. 2018;33:1419–27.
Mintziras I, Miligkos M, Wächter S, Manoharan J, Maurer E, Bartsch DK. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: systematic review and meta-analysis. Int J Surg. 2018;59:19–26.
Findlay M, Brown C, De Abreu Lourenço R, White K, Bauer J. Sarcopenia and myosteatosis in patients undergoing curative radiotherapy for head and neck cancer: impact on survival, treatment completion, hospital admission and cost. J Hum Nutr Diet. 2020;33:811–21.
Prado CM, Purcell SA, Laviano A. Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle. 2020;8:jcsm.12525.
Author information
Authors and Affiliations
Contributions
DLC, SJC and MF participated in study conception, design and acquisition/analysis of the data. All authors were involved in interpretation of the data, critical revision of the manuscript and approval of the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
DLC has received honoraria and travel support from Novartis and Ipsen, outside the scope of the submitted work. NP has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Roche Pharma AG, Merck Serono and Ipsen and has consulted for Merck Serono, Amgen, Bayer HealthCare Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Pfizer, Novartis, Roche Pharma AG, Ipsen, Merck, Baxalta, Specialised Therapeutics and AstraZeneca, outside the scope of the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chan, D.L., Clarke, S.J., Engel, A. et al. Computed tomography (CT)-defined sarcopenia and myosteatosis are prevalent in patients with neuroendocrine neoplasms (NENs) treated with peptide receptor radionuclide therapy (PRRT). Eur J Clin Nutr 76, 143–149 (2022). https://doi.org/10.1038/s41430-021-00915-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-021-00915-4
This article is cited by
-
Sarcopenia and Neuroendocrine Neoplasms
Current Oncology Reports (2024)